| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
KDM5
|
|---|---|
| 体外研究 (In Vitro) |
用正常化疗或靶向药物治疗的几种癌细胞系模型表明,CPI-455 减少了 DTP 的量,提高了 H3K4 三甲基化 (H3K4me3) 的总体水平,并促进 KDM5 抑制 [1]。 CPI-455 对目标 KDM5 蛋白具有高亲和力。在接触两种活性药物 CPI-455 和 CPI-766 中的任何一种后 24 小时内,H3K4me3 出现剂量依赖性升高。计算出三种管腔乳腺癌细胞系 MCF-7、T-47 和 EFM-19 对 KDM5 抑制剂 CPI0455 的 IC50 值分别为 35.4、26.19 和 16.13 μM [2]。
|
| 体内研究 (In Vivo) |
用 CPI-455(50/70 mg/kg,腹腔注射,每日)治疗双重阻断 B7-H4 和 KDM5B 的小鼠产生了保护性免疫 [2]。
|
| 酶活实验 |
组蛋白去甲基酶的KDM5家族催化组蛋白H3在赖氨酸4(H3K4)上的去甲基化,并且是药物耐受性癌症持续细胞(DTPs)生存所需的。在这里,我们报告了特异性KDM5抑制剂CPI-455的发现和特征。KDM5A的晶体结构揭示了CPI-455的抑制机制以及影响底物结合的蛋白质结构域的拓扑排列。CPI-455介导的KDM5抑制,提高了H3K4三甲基化(H3K4me3)的总体水平,并降低了用标准化疗或靶向药物治疗的多个癌症细胞系模型中的DTP数量。这些发现表明,用KDM5-特异性抑制剂预处理癌症细胞会导致癌症细胞亚群的消融,这些细胞亚群可以作为治疗复发的基础[1]。
|
| 细胞实验 |
Chemotaxis assay/趋化性测定[3]
使用磁珠通过阴性选择从PBMC中选择CD8+淋巴细胞,并与抗CD3/抗CD28包被的磁珠一起培养7天,以产生CD8+效应细胞。这些细胞被装载到transwell插入物的上部腔室中(孔径为5.0-μm)。在底部孔中,加入含有不同量的趋化因子中和抗体的培养基,或来自牙龈卟啉单胞菌感染细胞系(Kyse-410和Kyse-150)的培养上清液。使用KDM5B抑制剂CPI-455。对于抗体阻断试验,将中和抗CXCL9(MAB392)、抗CXCL10(MAB266)和抗CXCL11加入培养上清液中,在37°C下孵育30分钟,然后加入T细胞。收集下腔内容物,通过FACS测定CD8+细胞的百分比。 |
| 动物实验 |
Animal/Disease Models: Sixweeks old male C57BL/6 mice (One- to 2-mm fragments of P. gingivalis–positive PDXs were implanted subcutaneously (sc) into the flank region of humanized mice.)
Doses: 50 mg/kg or 70 mg/ kg (combined with anti–B7-H4). Route of Administration: IP, daily, 14-28 days. Experimental Results: Histopathology analysis revealed no inflammation in either group at 2 weeks in response to the primary infection. However, at 8 weeks after inoculation, mice receiving monotherapy demonstrated mild inflammation, whereas the combined treatment presented with heavy to severe inflammation, which persisted at 12 and 16 weeks after challenge. Treatment with CPI-455 to selectively target H3K4-specific JmjC demethylases increased CXCL11, CXCL9, and CXCL10 following infection , with maximum levels observed 48 hrs (hours) after infection. In vivo tumor studies[3] One- to 2-mm fragments of P. gingivalis–positive PDXs were implanted subcutaneously into the flank region of humanized mice. After injection, the mice were randomly divided into different groups (n = 10/group). Mice were treated with CPI-455 (50 mg/kg or 70 mg/kg, daily, intraperitoneal injection) and anti–B7-H4 (188; 500 μg/mouse, weekly, intraperitoneal injection), followed by the sequential administration of CPI-455 and anti–B7-H4 Ab started on days 6 and 20, respectively; phased combined treatment for 14 days with CPI-455 started on day 6 and combined treatment with anti–B7-H4 Ab started on day 13 and continuing for 3 weeks; or extended phased combined treatment with CPI-455 for 28 days. The animals dosed according to the appropriate schema (n = 10 mice/group) were monitored daily for up to 2 months, and the objective response rate and survival were recorded.[3] An additional cohort of mice (n = 5/group) was included to conduct mechanistic studies. In this cohort, the mice were sacrificed on day 30 after tumor inoculation. Residual tumors were surgically removed before terminal escape (tumor with partial response, PR) or complete remission (tumor with complete response) and processed for IHC and flow cytometry analysis. IHC and flow cytometry results related to lymphocyte infiltration were determined, and a representative mouse from each treatment group [(i) animals receiving control therapy, (ii) animals receiving anti–B7-H4 Ab monotherapy, (iii) animals receiving 75 mg/kg CPI-455 monotherapy, and (iv) animals treated with extended phased therapy using 75 mg/kg dose CPI-455] in this separate cohort is shown, TV (mm3) = π/6 × length × width2. Mice suffering from progressive disease or those used for subsequent analysis were euthanized when the TV was more than 2,500 mm3. |
| 参考文献 | |
| 其他信息 |
Pathogens are capable of hijacking immune defense mechanisms, thereby creating a tolerogenic environment for hypermutated malignant cells that arise within the site of infection. Immune checkpoint-oriented immunotherapies have shown considerable promise. Equally important, the epigenetic reprogramming of an immune-evasive phenotype that activates the immune system in a synergistic manner can improve immunotherapy outcomes. These advances have led to combinations of epigenetic- and immune-based therapeutics. We previously demonstrated that Porphyromonas gingivalis isolated from esophageal squamous cell carcinoma (ESCC) lesions represents a major pathogen associated with this deadly disease. In this study, we examined the mechanisms associated with host immunity during P. gingivalis infection and demonstrated that experimentally infected ESCC responds by increasing the expression of B7-H4 and lysine demethylase 5B, which allowed subsequent in vivo analysis of the immunotherapeutic effects of anti-B7-H4 and histone demethylase inhibitors in models of chronic infection and immunity against xenografted human tumors. Using three different preclinical mouse models receiving combined therapy, we showed that mice mounted strong resistance against P. gingivalis infection and tumor challenge. This may have occurred via generation of a T cell-mediated response in the microenvironment and formation of immune memory. In ESCC subjects, coexpression of B7-H4 and KDM5B correlated more significantly with bacterial load than with the expression of either molecule alone. These results highlight the unique ability of P. gingivalis to evade immunity and define potential targets that can be exploited therapeutically to improve the control of P. gingivalis infection and the development of associated neoplasia.[3]
|
| 分子式 |
C16H15CLN4O
|
|
|---|---|---|
| 分子量 |
314.77
|
|
| 精确质量 |
278.116
|
|
| 元素分析 |
C, 69.05; H, 5.07; N, 20.13; O, 5.75
|
|
| CAS号 |
1628208-23-0
|
|
| 相关CAS号 |
CPI-455 hydrochloride;2095432-28-1
|
|
| PubChem CID |
78426698
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
488.7±55.0 °C at 760 mmHg
|
|
| 闪点 |
249.3±31.5 °C
|
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
|
| 折射率 |
1.670
|
|
| LogP |
2.46
|
|
| tPSA |
68.5
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
21
|
|
| 分子复杂度/Complexity |
611
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
0
|
|
| InChi Key |
VGXRQCOVGLGFIM-UHFFFAOYSA-N
|
|
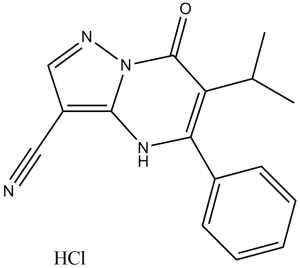
| InChi Code |
InChI=1S/C16H14N4O/c1-10(2)13-14(11-6-4-3-5-7-11)19-15-12(8-17)9-18-20(15)16(13)21/h3-7,9-10,18H,1-2H3
|
|
| 化学名 |
7-oxo-5-phenyl-6-propan-2-yl-1H-pyrazolo[1,5-a]pyrimidine-3-carbonitrile
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1769 mL | 15.8846 mL | 31.7692 mL | |
| 5 mM | 0.6354 mL | 3.1769 mL | 6.3538 mL | |
| 10 mM | 0.3177 mL | 1.5885 mL | 3.1769 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
 Identification and characterization of a potent and selective KDM5 inhibitor with reversible activity in cells.Nat Chem Biol.2016 Jul;12(7):531-8. |
|---|
 CPI-455 selectively affects H3K4 methylation in several cell models.Nat Chem Biol.2016 Jul;12(7):531-8. |
 Crystal structure of KDM5A.Nat Chem Biol.2016 Jul;12(7):531-8. |
 Inhibitor binding at the KDM5A active site. |
|---|
 KDM5 activity is increased in DTP modelsin vitroandin vivo.Nat Chem Biol.2016 Jul;12(7):531-8. |
 KDM5 inhibition suppresses the emergence of drug-tolerant cells.Nat Chem Biol.2016 Jul;12(7):531-8. |