| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
(Z)-噻吩具有防止细胞死亡和/或增加细胞存活和/或可塑性的能力,特别是在细胞(包括神经细胞)中存在有毒物质的情况下[1]。噻吩的 Z(顺式)异构体被称为(Z)-噻吩,它作为合成前体和降解剂 [2]。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Fifty-nine plasma thiothixene concentrations were measured in 42 patients as part of routine therapeutic drug monitoring. Data collection included concomitant medications, smoking history, and demographic variables. A retrospective analysis was performed to assess the effect of these parameters on oral thiothixene clearance. When groups of patients were categorized by concomitant medications (i.e., no interacting drugs, enzyme/clearance inducers, and enzyme/clearance inhibitors), thiothixene clearance was found to be significantly increased by enzyme inducing drugs (e.g., anticonvulsants) and decreased by clearance inhibiting agents (e.g., cimetidine). Tobacco smoking significantly increased the hepatic clearance of thiothixene within the no interactions and inhibitor groups, but not in the inducer group. Significantly more patients in the inducer group had nondetectable plasma concentrations of thiothixene than the other groups. When the entire patient population was dichotomized by age, patients less than 50 years old had a significantly greater mean clearance (48.2 +/- 37.8 liters/min) versus those greater than or equal to 50 (20.0 +/- 12.6 liters/min). Men in this cohort exhibited a significantly higher clearance (49.2 +/- 38.7 liters/min) than did the women (22.0 +/- 13.5 liters/min). By taking into account these potential sources of pharmacokinetic variability when monitoring plasma thiothixene concentrations, more appropriate dosing of thiothixene may be achieved. Controlled, prospective studies are needed to validate these findings. Thiothixene is widely distributed into body tissues and may remain in the body for several weeks following administration. Thiothixene is well absorbed from the GI tract. Therapeutic response may occur within a few days to several weeks following oral administration of the drug. Plasma concentrations required for therapeutic effects are not known. Two experiments are reported in which acute single test dose levels of thiothixene (Navane) were correlated with age. In the first study 20 mg oral doses were given to 28 male subjects and serum levels were drawn 2 hr later. Mean age was 30 and correlation of serum level with age was 0.43, P less than 0.02. In a second older group with a mean age of 41, 10 mg oral doses were given to 25 subjects. A correlation with age of 0.41, P less than 0.05 was obtained with age. In prior work such acute levels have been found to correlate with steady-state serum levels and with clinical response to the medication. ... Metabolism / Metabolites Hepatic. Thiothixene is metabolized in the liver and is excreted mainly in feces via biliary elimination as unchanged drug and as the demethyl, sulfoxide, demethylated sulfoxide, and hydroxylated thiothixene derivatives. Biological Half-Life 10-20 hours |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Thiothixene is a solid. It is antipsychotic agent and dopamine antagonist. Thiothixene capsules are effective in the management of schizophrenia. HUMAN STUDIES: Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with antipsychotic drugs, including thiothixene. A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with antipsychotic drugs, including thiothixene. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmias). Manifestations of overdose include muscular twitching, drowsiness and dizziness. Symptoms of gross overdosage may include CNS depression, rigidity, weakness, torticollis, tremor, salivation, dysphagia, hypotension, disturbances of gait, or coma. Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Thiothixene may be additive with or may potentiate the action of other CNS depressants (including alcohol), anticholinergics, or hypotensive agents. ANIMAL STUDIES: In animal reproduction studies with thiothixene, there was some decrease in conception rate and litter size, and an increase in resorption rate in rats and rabbits. After repeated oral administration of thiothixene to rats (5 to 15 mg/kg/day), rabbits (3 to 50 mg/kg/day), and monkeys (1 to 3 mg/kg/day) before and during gestation, no teratogenic effects were seen. Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Because there is no published experience with thiothixene during breastfeeding, other antipsychotic agents are preferred. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Thiothixene has caused galactorrhea. Hyperprolactinemia appears to be the cause of the galactorrhea. The hyperprolactinemia is caused by the drug's dopamine-blocking action in the tuberoinfundibular pathway. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Interactions Hepatic microsomal enzyme inducing agents, such as carbamazepine, were found to significantly increase the clearance of thiothixene. Patients receiving these drugs should be observed for signs of reduced thiothixene effectiveness. In this study healthy volunteers received thiothixene with and without a 3-day pretreatment with paroxetine to determine if paroxetine decreased the clearance of thiothixene. Ten healthy medication-free volunteers (4 women and 6 men, mean age 38 +/- 12 years) were randomized to receive a single 20 mg oral dose of thiothixene on two separate occasions. On one occasion thiothixene was given concurrently, and following 3 days of pre-treatment with oral paroxetine (20 mg/day). On the other occasion thiothixene was given without paroxetine pre-treatment. The two study days were separated by a minimum period of 2 weeks. On both study days, after the administration of thiothixene, 10 mL blood samples were collected over the next 72 hr. None of the pharmacokinetic parameters of thiothixene were significantly altered by a 3-day treatment with paroxetine. It is likely that the CYP2D6 isoenzyme is not responsible for a high proportion of thiothixene clearance, but one cannot exclude the possibility that a longer paroxetine pretreatment might have caused some inhibition of thiothixene clearance. Thiothixene may be additive with or may potentiate the action of other CNS depressants (including alcohol), anticholinergics, or hypotensive agents. Due to a possible additive effect with hypotensive agents, patients receiving these drugs should be observed closely for signs of excessive hypotension when thiothixene is added to their drug regimen. Non-Human Toxicity Values LD50 Rat oral 720 mg/kg LD50 Rat sc 2 g/kg LD50 Mouse oral 400 mg/kg LD50 Mouse sc 4 g/kg |
| 参考文献 | |
| 其他信息 |
Thiothixene is a N-methylpiperazine. It has a role as an anticoronaviral agent.
A thioxanthine used as an antipsychotic agent. Its effects are similar to the phenothiazine antipsychotics. Thiothixene is a Typical Antipsychotic. Thiothixene is a thioxanthene derivative and a dopamine antagonist with antipsychotic property. Thiothixene blocks postsynaptic dopamine receptors in the mesolimbic system and medullary chemoreceptor trigger zone, thereby decreasing dopamine activity leading to decreased stimulation of the vomiting center and psychotic effects, such as hallucinations and delusions. In addition, this agent blocks the D2 somatodendritic autoreceptor, thereby increasing dopamine turnover. Thiothixene possesses weak affinity for the histamine H1 and alpha-adrenergic receptors. Thiothixene Hydrochloride is the hydrochloride salt form of thiothixene, a thioxanthene derivative and a dopamine antagonist with antipsychotic property. Thiothixene blocks postsynaptic dopamine receptors in the mesolimbic system and medullary chemoreceptor trigger zone, thereby decreasing dopamine activity leading to decreased stimulation of the vomiting center and psychotic effects, such as hallucinations and delusions. In addition, this agent blocks the D2 somatodendritic autoreceptor, thereby increasing dopamine turnover. Thiothixene possesses weak affinity for the histamine H1 and alpha-adrenergic receptors. A thioxanthine used as an antipsychotic agent. Its effects are similar to the phenothiazine antipsychotics. See also: Thiothixene Hydrochloride (has salt form). Drug Indication For the management of schizophrenia. Mechanism of Action Thiothixene acts as an antagonist (blocking agent) on different postsysnaptic receptors -on dopaminergic-receptors (subtypes D1, D2, D3 and D4 - different antipsychotic properties on productive and unproductive symptoms), on serotonergic-receptors (5-HT1 and 5-HT2, with anxiolytic, antidepressive and antiaggressive properties as well as an attenuation of extrapypramidal side-effects, but also leading to weight gain, fall in blood pressure, sedation and ejaculation difficulties), on histaminergic-receptors (H1-receptors, sedation, antiemesis, vertigo, fall in blood pressure and weight gain), alpha1/alpha2-receptors (antisympathomimetic properties, lowering of blood pressure, reflex tachycardia, vertigo, sedation, hypersalivation and incontinence as well as sexual dysfunction, but may also attenuate pseudoparkinsonism - controversial) and finally on muscarinic (cholinergic) M1/M2-receptors (causing anticholinergic symptoms like dry mouth, blurred vision, obstipation, difficulty/inability to urinate, sinus tachycardia, ECG-changes and loss of memory, but the anticholinergic action may attenuate extrapyramidal side-effects). Therapeutic Uses Antipsychotic Agents; Dopamine Antagonists /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Thiothixene is included in the database. Thiothixene capsules are effective in the management of schizophrenia. /Included in US product label/ Experience with the drug in treating neurotic conditions is limited and does not indicate that thiothixene is likely to have advantages over anxiolytic agents, butyrophenones, phenothiazines, or chlorprothixene (no longer commercially available in the US). Thiothixene has not been evaluated in the management of behavioral complications in mentally retarded patients. Drug Warnings /BOXED WARNING/ Increased Mortality in Elderly Patients with Dementia-Related Psychosis: Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Thiothixene is not approved for the treatment of patients with dementia-related psychosis. Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with antipsychotic drugs, including thiothixene. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of antipsychotic treatment, which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown. A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with antipsychotic drugs, including thiothixene. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmias). The most frequent adverse effects of thiothixene are drowsiness (which usually is mild and subsides with continuation of therapy) and extrapyramidal symptoms. Like propylpiperazine phenothiazines, thiothixene is more likely to produce akathisia and dystonia than parkinson-like syndromes. Generally, extrapyramidal effects can be controlled by reducing the dosage of thiothixene and/or administering an antiparkinsonian drug. For more Drug Warnings (Complete) data for Thiothixene (21 total), please visit the HSDB record page. Pharmacodynamics Thiothixene is an antipsychotic of the thioxanthene series. Navane possesses certain chemical and pharmacological similarities to the piperazine phenothiazines and differences from the aliphatic group of phenothiazines. Although widely used in the treatment of schizophrenia for several decades, thiothixene is seldom used today in favor of atypical antipsychotics such as risperidone. |
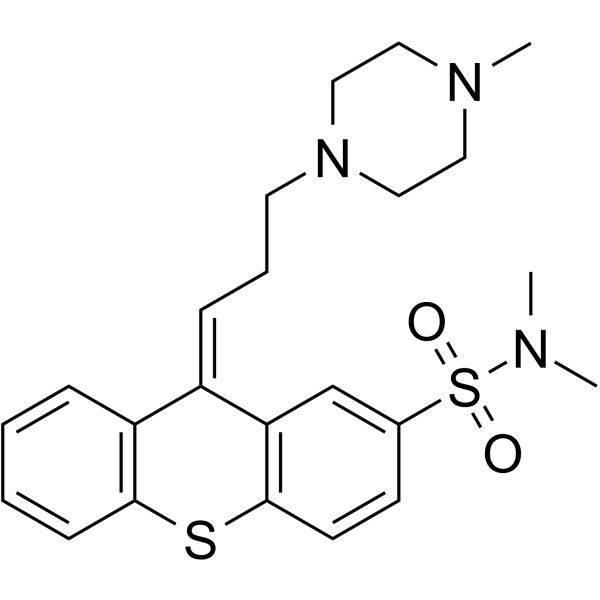
| 分子式 |
C23H29N3O2S2
|
|---|---|
| 分子量 |
443.62526
|
| 精确质量 |
443.17
|
| CAS号 |
3313-26-6
|
| 相关CAS号 |
Thiothixene hydrochloride;49746-04-5
|
| PubChem CID |
941651
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.269 g/cm3
|
| 沸点 |
599ºC at 760 mmHg
|
| 熔点 |
114-118ºC
|
| 闪点 |
316.1ºC
|
| 折射率 |
1.643
|
| LogP |
4.427
|
| tPSA |
77.54
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
30
|
| 分子复杂度/Complexity |
711
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CN1CCN(CC1)CC/C=C\2/C3=CC=CC=C3SC4=C2C=C(C=C4)S(=O)(=O)N(C)C
|
| InChi Key |
GFBKORZTTCHDGY-UWVJOHFNSA-N
|
| InChi Code |
InChI=1S/C23H29N3O2S2/c1-24(2)30(27,28)18-10-11-23-21(17-18)19(20-7-4-5-9-22(20)29-23)8-6-12-26-15-13-25(3)14-16-26/h4-5,7-11,17H,6,12-16H2,1-3H3/b19-8-
|
| 化学名 |
(9Z)-N,N-dimethyl-9-[3-(4-methylpiperazin-1-yl)propylidene]thioxanthene-2-sulfonamide
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~10 mg/mL (~22.54 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1 mg/mL (2.25 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 10.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1 mg/mL (2.25 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 10.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 1 mg/mL (2.25 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2541 mL | 11.2707 mL | 22.5413 mL | |
| 5 mM | 0.4508 mL | 2.2541 mL | 4.5083 mL | |
| 10 mM | 0.2254 mL | 1.1271 mL | 2.2541 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。