| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
nAChR (IC50 = 8.9 μM)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:长春花碱的平均终末半衰期为 14.3 小时。当在新鲜分离的大鼠肝细胞中孵育时,VLB 可能通过被动扩散机制以及随后的紧密细胞结合快速而强烈地渗透到细胞中。长春花碱抑制肾上腺髓质素诱导的血管生成反应,并且对有丝分裂滑移呈阳性反应,导致单核细胞中的微核具有胞质分裂阻滞。根据 RPD、RICC 和 RCC 的计算,长春花碱在导致约 50% 细胞死亡和细胞抑制或更少的浓度下显着增加微核单核细胞。细胞测定:设置六孔处理板,每孔含有 5 × 104 个细胞/mL(中国仓鼠卵巢(CHO)细胞),悬浮于 3 mL 培养基中,用长春花碱处理 3 h,然后21小时生长。
|
||
| 体内研究 (In Vivo) |
长春花碱是一种广泛使用的抗癌药物,具有不良副作用。极低剂量的VBL和RAP组合对抗人HCC在体内获得了令人满意的抗血管生成作用。临床相关剂量的长春花碱可抑制体内 CEM 细胞中微管蛋白的棕榈酰化(对微管蛋白去棕榈酰化的影响)。
|
||
| 细胞实验 |
六孔处理板每孔制备 5 × 104 细胞/mL,悬浮于 3 mL 培养基中,用长春花碱处理 3 小时,随后生长 21 个周期。小时。
|
||
| 动物实验 |
|
||
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Most sources consider breastfeeding to be contraindicated during maternal antineoplastic drug therapy. It is probably impractical to resume breastfeeding after vinblastine therapy because of the drug's long half-life. Chemotherapy may adversely affect the normal microbiome and chemical makeup of breastmilk. Women who receive chemotherapy during pregnancy are more likely to have difficulty nursing their infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk A woman diagnosed with Hodgkin's lymphoma during the second trimester of pregnancy received 3 rounds of chemotherapy during the third trimester of pregnancy and resumed chemotherapy 4 weeks postpartum. Milk samples were collected 15 to 30 minutes before and after chemotherapy for 16 weeks after restarting. The regimen consisted of doxorubicin 40 mg, bleomycin 16 units, vinblastine 9.6 mg and dacarbazine 600 mg, all given over a 2-hour period every 2 weeks. The microbial population and metabolic profile of her milk were compared to those of 8 healthy women who were not receiving chemotherapy. The breastmilk microbial population in the patient was markedly different from that of the healthy women, with increases in Acinetobacter sp., Xanthomonadacae and Stenotrophomonas sp. and decreases in Bifidobacterium sp. and Eubacterium sp. Marked differences were also found among numerous chemical components in the breastmilk of the treated woman, most notably DHA and inositol were decreased. A telephone follow-up study was conducted on 74 women who received cancer chemotherapy at one center during the second or third trimester of pregnancy to determine if they were successful at breastfeeding postpartum. Only 34% of the women were able to exclusively breastfeed their infants, and 66% of the women reported experiencing breastfeeding difficulties. This was in comparison to a 91% breastfeeding success rate in 22 other mothers diagnosed during pregnancy, but not treated with chemotherapy. Other statistically significant correlations included: 1. mothers with breastfeeding difficulties had an average of 5.5 cycles of chemotherapy compared with 3.8 cycles among mothers who had no difficulties; and 2. mothers with breastfeeding difficulties received their first cycle of chemotherapy on average 3.4 weeks earlier in pregnancy. Of the 6 women who received a vincristine-containing regimen, 5 had breastfeeding difficulties. |
||
| 参考文献 |
|
||
| 其他信息 |
Vinblastine Sulfate can cause developmental toxicity according to state or federal government labeling requirements.
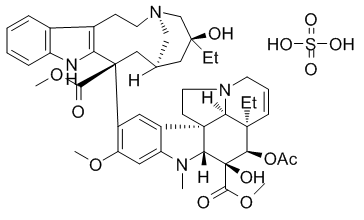
Vinblastine sulfate appears as an anticancer drug. White to slightly yellow crystalline powder. (NTP, 1992) Vinblastine Sulfate is the sulfate salt of vinblastine, a natural alkaloid isolated from the plant Catharanthus roseus (Madagascar periwinkle) with antineoplastic properties. Vinblastine disrupts microtubule formation and function during mitosis and interferes with glutamic acid metabolism. (NCI04) Antitumor alkaloid isolated from Vinca rosea. (Merck, 11th ed.) See also: Vinblastine (has active moiety). |
| 分子式 |
C46H60N4O13S
|
|---|---|
| 分子量 |
909.05
|
| 精确质量 |
908.387
|
| 元素分析 |
C, 60.78; H, 6.65; N, 6.16; O, 22.88; S, 3.53
|
| CAS号 |
143-67-9
|
| 相关CAS号 |
143-67-9 (sulfate);865-21-4 (free);
|
| PubChem CID |
5388983
|
| 外观&性状 |
White to slight yellow solid powder
|
| 密度 |
1.37 g/cm3
|
| 熔点 |
267 °C (dec.)(lit.)
|
| LogP |
4.359
|
| tPSA |
237.08
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
16
|
| 可旋转键数目(RBC) |
10
|
| 重原子数目 |
64
|
| 分子复杂度/Complexity |
1780
|
| 定义原子立体中心数目 |
9
|
| SMILES |
S(=O)(=O)(O[H])O[H].O(C(C([H])([H])[H])=O)[C@@]1([H])[C@](C(=O)OC([H])([H])[H])([C@@]2([H])C3(C4=C([H])C([C@]5(C(=O)OC([H])([H])[H])C6=C(C7=C([H])C([H])=C([H])C([H])=C7N6[H])C([H])([H])C([H])([H])N6C([H])([H])[C@](C([H])([H])C([H])([H])[H])(C([H])([H])[C@]([H])(C6([H])[H])C5([H])[H])O[H])=C(C([H])=C4N2C([H])([H])[H])OC([H])([H])[H])C([H])([H])C([H])([H])N2C([H])([H])C([H])=C([H])[C@]1(C([H])([H])C([H])([H])[H])[C@]23[H])O[H]
|
| InChi Key |
KDQAABAKXDWYSZ-JKDPCDLQSA-N
|
| InChi Code |
InChI=1S/C46H58N4O9.H2O4S/c1-8-42(54)23-28-24-45(40(52)57-6,36-30(15-19-49(25-28)26-42)29-13-10-11-14-33(29)47-36)32-21-31-34(22-35(32)56-5)48(4)38-44(31)17-20-50-18-12-16-43(9-2,37(44)50)39(59-27(3)51)46(38,55)41(53)58-7;1-5(2,3)4/h10-14,16,21-22,28,37-39,47,54-55H,8-9,15,17-20,23-26H2,1-7H3;(H2,1,2,3,4)/t28-,37+,38-,39-,42+,43-,44-,45+,46+;/m1./s1
|
| 化学名 |
methyl (1R,9R,10S,11R,12R,19R)-11-acetyloxy-12-ethyl-4-[(13S,15S,17S)-17-ethyl-17-hydroxy-13-methoxycarbonyl-1,11-diazatetracyclo[13.3.1.04,12.05,10]nonadeca-4(12),5,7,9-tetraen-13-yl]-10-hydroxy-5-methoxy-8-methyl-8,16-diazapentacyclo[10.6.1.01,9.02,7.016,19]nonadeca-2,4,6,13-tetraene-10-carboxylate;sulfuric acid
|
| 别名 |
Vincaleucoblastine; VLB; Velsar; Velban
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~100 mg/mL (~110 mM)
Water: ~50 mg/mL (~55 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (2.75 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (2.75 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (2.75 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 50 mg/mL (55.00 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.1000 mL | 5.5002 mL | 11.0005 mL | |
| 5 mM | 0.2200 mL | 1.1000 mL | 2.2001 mL | |
| 10 mM | 0.1100 mL | 0.5500 mL | 1.1000 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Pembrolizumab and Combination Chemotherapy Before Surgery for the Treatment of Muscle-Invasive Bladder Cancer
CTID: NCT04383743
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-06-27