| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
Glycopeptide
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:万古霉素是一种大糖肽化合物,分子量为1450 Da。万古霉素是一种独特的糖肽,在结构上与任何目前可用的抗生素无关。它还具有独特的作用方式,抑制敏感细菌细胞壁合成的第二阶段。万古霉素对多种革兰氏阳性菌具有活性,例如金黄色葡萄球菌、葡萄球菌。表皮,Str。无乳链,Str。博维斯海峡变形链球菌、草绿色链球菌、肠球菌。
|
| 体内研究 (In Vivo) |
万古霉素通过静脉注射,标准输注时间至少为 1 小时,以尽量减少输注相关的不良反应。对于肌酐清除率正常的受试者,万古霉素的 α-分布期为 30 分钟至 1 小时,β-消除半衰期为 6-12 小时。分布容积为0.4-1 L/kg。万古霉素与蛋白质的结合率为 10% 至 50%。影响万古霉素整体活性的因素包括其组织分布、接种量和蛋白质结合效应。万古霉素对感染小鼠的治疗与临床、腹泻和组织病理学评分以及治疗期间生存率的改善有关。
|
| 酶活实验 |
万古霉素是一种独特的糖肽,在结构上与目前可用的任何抗生素都无关。它还有一种独特的作用模式,可以抑制易感细菌细胞壁合成的第二阶段。还有证据表明,万古霉素改变了细胞膜的通透性,并选择性地抑制核糖核酸的合成。用万古霉素从易感生物体中诱导细菌L相变体是极其困难的,并且这种变体是不稳定的。由其他药物诱导的稳定L相变体对万古霉素敏感。万古霉素对大量革兰氏阳性菌具有活性,如金黄色葡萄球菌(包括耐甲氧西林菌株)、葡萄球菌。表皮葡萄球菌(包括多重耐药菌株)、肺炎链球菌(包括多重耐药性菌株)、化脓性链球菌、无乳链球菌、牛链球菌、变形链球菌、绿色链球菌、肠球菌、梭菌属、白喉杆菌、单核细胞增生李斯特菌、放线菌属和乳杆菌属。在过去的三十年里,对万古霉素的耐药性没有增加。万古霉素和一种氨基糖苷类药物联合对抗葡萄球菌的抗菌活性得到了增强。金黄色葡萄球菌、牛链球菌、肠球菌和绿色链球菌。万古霉素和利福平的组合对大多数葡萄球菌菌株具有拮抗作用。金黄色葡萄球菌,虽然表现出冷漠和偶尔的协同作用,但对葡萄球菌菌株具有协同作用。表皮病。它显示出对肠球菌的漠不关心。万古霉素和fusidic酸对葡萄球菌无明显作用。金黄色葡萄球菌[2]。
|
| 细胞实验 |
C.艰难梭菌毒素测定。艰难梭菌毒素A和B使用Tech Lab毒素A/B II ELISA试剂盒的改良方案进行检测。对每个粪便样品进行称重,并将每个样品的稀释剂量标准化,以提供每个样品相同的粪便质量与稀释剂的比例。通过研磨和涡流将稀释剂-样品混合物均化,并对样品进行1:10、1:100和1:1000系列稀释。将每个样品的1:1000稀释液总计150μl添加到试剂盒中提供的预涂孔中。阴性对照由150μl稀释剂组成,阳性对照由135μl稀释剂加3滴试剂盒中提供的阳性对照毒素A-B混合物组成。向每个孔中加入一滴缀合物,并将平板在37°C下孵育50分钟。用试剂盒中提供的150μl 1倍稀释液洗涤每个孔三次。向每个孔中加入两滴基质。10分钟后,向每个孔中加入1滴停止溶液。在ELISA读取器中读取之前,将板放置2分钟[3]。
|
| 动物实验 |
Mice: In one series of studies, infected mice are given either vancomycin (20 mg/kg) daily for five or ten days and monitored for fifteen days after infection, or vancomycin (50 mg/kg) daily for one, two, three, or five days and monitored for twenty-one days after infection[3].
Murine model of C. difficile infection and treatment.[3] The infection model is a modification of the published protocol of Chen et al. This protocol has been approved by the Center for Comparative Medicine at University of Virginia. C57BL/6 mice, male, 8 weeks old, were used. From 6 to 4 days prior to infection, mice were given an antibiotic cocktail containing vancomycin (0.0045 mg/g), colistin (0.0042 mg/g), gentamicin (0.0035 mg/g), and metronidazole (0.0215 mg/g) in drinking water. One day prior to infection, clindamycin (32 mg/kg of body weight) was injected subcutaneously. The mice were divided into the following groups: control uninfected, control infected, infected and treated with vancomycin (20 mg/kg), and infected and treated with comparator drugs—nitazoxanide, fidaxomicin, and metronidazole (all drugs given at 20 mg/kg/day). Food and water were allowed ad libitum. Although each mouse or treatment group was housed in a separate cage, all mice were housed in the same pod of the vivarium. Infection was performed with VPI 10463 (ATCC) as an inoculum of 104 or 105 administered by oral gavage. This strain produces both C. difficile toxins A (TcdA) and B (TcdB). One day postinfection, treated mice were given either vancomycin or nitazoxanide at 20 mg/kg each by oral gavage daily for 5 days and monitored for either 1 or 2 weeks postinfection. One set of experiments was performed in which infected mice were treated with vancomycin (50 mg/kg) daily for 1, 2, 3, or 5 days and were observed for 21 days postinfection or with vancomycin (20 mg/kg) daily for either 5 or 10 days and monitoring for 15 days postinfection. In a separate experiment, mice given a preinfection antibiotic regimen described above were treated with either vancomycin, fidaxomicin, or metronidazole at 20 mg/kg/day for 5 days and infected another 5 days later. Except when indicated, all comparator drugs were administered using the same dosage (20 mg/kg/day for 5 days) to equally compare efficacies, outcomes, and effects on selected gut floras between treatment groups as previously described. From another study, a group of control mice was given vancomycin but was not infected. A clinical scoring system was developed on the basis of weight loss, diarrhea, activity level, and appearance of eyes and hair (each parameter scored from 0 to 3, where 0 is normal and 3 is the worst; maximum score of 20). Stool specimens were collected daily. Diarrhea was scored as follows: 1 for soft or color change (yellow), 2 for wet tail or mucoid, and 3 for liquid or no stool (ileus). Mice judged moribund by the clinical score (score of >14) at any day and all surviving mice at the end of the experiment were sacrificed, and intestinal tissues and cecal contents were collected as described below. A separate set of experiments was performed for harvesting cecal contents for clostridial bacterial and toxin burdens at days 3, 6, 9, and 12 to 13 postinfection to follow changes at different time points of the study. Histopathology. Upon euthanasia, cecal and colonic tissues were fixed in 10% zinc formalin overnight and then placed in 10% ethanol before being sent for paraffin embedding and hematoxylin and eosin (H&E) staining at the University of Virginia Histology Research Core. Histopathologic scoring was performed coded (C.A.W. and M.S.R.). H&E-stained tissues were scored for mucosal disruption, mucosal hypertrophy, inflammation, vascular congestion and exudates, and submucosal edema (each parameter was graded from 0 to 3, with 0 as normal and 3 worst; maximum score of 15) as we previously described in detail. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Poorly absorbed from gastrointestinal tract, however systemic absorption (up to 60%) may occur following intraperitoneal administration. In the first 24 hours, about 75-80% of an administered dose of vancomycin is excreted in urine by glomerular filtration. The volume of distribution, as discussed in the literature, varies between 0.4-1 L/kg. The mean plasma clearance of vancomycin is about 0.058 L/kg/h. Vancomycin hydrochloride is not appreciably absorbed from the GI tract in most patients and must be given parenterally for the treatment of systemic infections. Oral bioavailability usually is less than 5%; however, limited data suggest that clinically important serum concentrations of the drug may result following enteral or oral administration of vancomycin in some patients with colitis and/or in those with renal impairment. In adults with normal renal function who received multiple 1 g doses of vancomycin (15 mg/kg) given by IV infusion over 1 hour, mean plasma concentrations immediately after completion of the infusion are approximately 63 ug/mL and mean plasma concentrations 2 and 11 hours later are approximately 23 or 8 ug/mL, respectively. When multiple 500-mg doses are given by IV infusion over 30 minutes, mean plasma concentrations are about 49 ug/mL immediately following the infusion and about 10 ug/mL 6 hours after infusion. Vancomycin is distributed into milk following IV administration. Systemic absorption of oral vancomycin is very low and it is not known whether the drug distributes into human milk following oral administration. Vancomycin readily crosses the placenta and is distributed into cord blood. Vancomycin is approximately 55% serum protein bound as measured by ultrafiltration at vancomycin serum concentrations of 10 to 100 mcg/mL. After IV administration of vancomycin hydrochloride, inhibitory concentrations are present in pleural, pericardial, ascitic, and synovial fluids; in urine; in peritoneal dialysis fluid; and in atrial appendage tissue. Vancomycin hydrochloride does not readily diffuse across normal meninges into the spinal fluid; but, when the meninges are inflamed, penetration into the spinal fluid occurs. Metabolism / Metabolites Since almost 75-80% of the drug is excreted unchanged in the urine after the first 24 hours following administration, there is seemingly no apparent metabolism of the drug. The concentration of vancomycin in the liver tissue and bile 24 hours after administration has also been reported at or below detection limits as well. Free toxin may be removed by opsonization via the reticuloendothelial system (primarily the liver and kidneys) or it may be degraded through cellular internalization via the lysosomes. Lysosomes are membrane-enclosed organelles that contain an array of digestive enzymes, including several proteases. Route of Elimination: In the first 24 hours, about 75% of an administered dose of vancomycin is excreted in urine by glomerular filtration. Half Life: Half-life in normal renal patients is approximately 6 hours (range 4 to 11 hours). In the first 24 hours, about 75% of an administered dose of vancomycin is excreted in urine by glomerular filtration. In anephric patients, the average half-life of elimination is 7.5 days. Biological Half-Life Half-life in normal renal patients is approximately 6 hours (range 4 to 11 hours). In anephric patients, the average half-life of elimination is 7.5 days. The mean elimination half-life of vancomycin from plasma is 4 to 6 hours in subjects with normal renal function. In anephric patients, the average half-life of elimination is 7.5 days. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
The bactericidal action of vancomycin results primarily from inhibition of cell-wall biosynthesis. Specifically, vancomycin prevents incorporation of N-acetylmuramic acid (NAM)- and N-acetylglucosamine (NAG)-peptide subunits from being incorporated into the peptidoglycan matrix; which forms the major structural component of Gram-positive cell walls. The large hydrophilic molecule is able to form hydrogen bond interactions with the terminal D-alanyl-D-alanine moieties of the NAM/NAG-peptides. Normally this is a five-point interaction. This binding of vancomycin to the D-Ala-D-Ala prevents the incorporation of the NAM/NAG-peptide subunits into the peptidoglycan matrix. In addition, vancomycin alters bacterial-cell-membrane permeability and RNA synthesis. There is no cross-resistance between vancomycin and other antibiotics. Vancomycin is not active in vitro against gram-negative bacilli, mycobacteria, or fungi. Toxicity Data LD50: 5000 mg/kg (Oral, Mouse) (A308) LD50: 319 mg/kg (Intravenous, Rat) (A308) LD50: 400 mg/kg (Intravenous, Mouse) (A308) Interactions Concomitant use of vancomycin and anesthetic agents has been associated with anaphylactoid reactions and an increased frequency of infusion reactions (e.g., hypotension, flushing, erythema, urticaria, pruritus). Erythema and histamine-like flushing has occurred in pediatric patients receiving vancomycin and anesthetic agents concomitantly. The risk of infusion-related adverse effects may be minimized if vancomycin is given as a 1-hour IV infusion prior to induction of anesthesia. In vitro, the antibacterial effects of vancomycin and aminoglycosides are synergistic against many strains of Staphylococcus aureus, nonenterococcal group D streptococci (Streptococcus bovis), enterococci (Enterococcus faecalis), and viridans streptococci. However, concomitant use of vancomycin and aminoglycosides is associated with an increased risk of ototoxicity and/or nephrotoxicity. Because of the possibility of additive toxicities, the concurrent or sequential systemic or topical use of other ototoxic and/or nephrotoxic drugs (e.g., aminoglycosides, amphotericin B, bacitracin, cisplatin, colistin, polymyxin B) and vancomycin requires careful serial monitoring of renal and auditory function. These drugs should be used with caution in patients receiving vancomycin therapy. Renal failure developed after a prolonged course of vancomycin therapy in 2 patients who were receiving tenofovir disoproxil fumarate as part of an antiretroviral regimen. Tenofovir has been implicated in the development of Fanconi syndrome and renal insufficiency because of its effects on the proximal renal tubule. Vancomycin nephrotoxicity is infrequent but may result from coadministration with a nephrotoxic agent. Clinicians should be aware that tenofovir may raise the risk of renal failure during prolonged administration of vancomycin. For more Interactions (Complete) data for Vancomycin (6 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mouse oral 5000 mg/kg /Monohydrochloride/ LD50 Mouse ip 1734 mg/kg LD50 Mouse iv 430 mg/kg LD50 Mouse sc 5000 mg/kg LD50 Rat iv 319 mg/kg |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Antibiotics, Glycopeptide Vancomycin hydrochloride is indicated for the treatment of serious or severe infections caused by susceptible strains of methicillin-resistant (beta-lactam-resistant) staphylococci. It is indicated for penicillin-allergic patients, for patients who cannot receive or who have failed to respond to other drugs, including the penicillins or cephalosporins, and for infections caused by vancomycin-susceptible organisms that are resistant to other antimicrobial drugs. Vancomycin hydrochloride is indicated for initial therapy when methicillin-resistant staphylococci are suspected, but after susceptibility data are available, therapy should be adjusted accordingly. /Included in US product label/ Vancomycin hydrochloride is effective in the treatment of staphylococcal endocarditis. Its effectiveness has been documented in other infections due to staphylococci, including septicemia, bone infections, lower respiratory tract infections, skin and skin-structure infections. When staphylococcal infections are localized and purulent, antibiotics are used as adjuncts to appropriate surgical measures. /Included in US product label/ The parenteral form of vancomycin hydrochloride may be administered orally for treatment of antibiotic-associated pseudomembranous colitis produced by C. difficile and for staphylococcal enterocolitis. Parenteral administration of vancomycin hydrochloride alone is of unproven benefit for these indications. Vancomycin hydrochloride is not effective by the oral route for other types of infection. /Included in US product label/ For more Therapeutic Uses (Complete) data for Vancomycin (12 total), please visit the HSDB record page. Drug Warnings Ototoxicity and nephrotoxicity are the most serious adverse effects of parenteral vancomycin therapy. The incidences of ototoxicity and nephrotoxicity have not been well established, but clinical experience to date suggests that these adverse effects occur relatively infrequently. Ototoxicity and nephrotoxicity are most likely to occur in patients with renal impairment, patients receiving IV vancomycin in high doses or for prolonged periods, or patients receiving other ototoxic and/or nephrotoxic drugs. Although ototoxicity and nephrotoxicity have been associated with serum or blood vancomycin concentrations of 80-100 ug/mL, these reactions have occurred with concentrations as low as 25 ug/mL. Correlations between serum vancomycin concentrations and ototoxicity and nephrotoxicity still remain to be clarified. Ototoxicity may be transient or permanent. Vancomycin may cause damage to the auditory branch of the eighth cranial nerve and permanent deafness has occurred. Vertigo, dizziness, and tinnitus have been reported rarely. Tinnitus may precede the onset of deafness and necessitates discontinuance of the drug. Deafness may progress despite cessation of vancomycin therapy. Vancomycin-induced nephrotoxicity may be manifested by transient elevations in BUN or serum creatinine concentrations, and the presence of hyaline and granular casts and albumin in the urine. Fatal uremia has occurred. Rarely, the drug has been associated with acute interstitial nephritis. Rapid IV administration of vancomycin has resulted in a hypotensive reaction frequently referred to as the "red-man syndrome" or "red-neck syndrome". The reaction is characterized by a sudden decrease in blood pressure which can be severe and may be accompanied by flushing and/or a maculopapular or erythematous rash on the face, neck, chest, and upper extremities; the latter manifestations may also occur in the absence of hypotension. Wheezing, dyspnea, angioedema, urticaria, and pruritus may also occur. Rarely, cardiac arrest or seizures have occurred. Vancomycin-induced hypotension appears to result from a negative inotropic and vasodilating action produced in part by a release of histamine, which is directly related to the rate of infusion; the release of histamine also appears to be responsible for the usual manifestations (e.g., erythema, rash, pruritus) of the "red" characterization. The reaction usually begins a few minutes after the vancomycin infusion is started, but may not occur until after the infusion is completed, and usually resolves spontaneously over one to several hours after discontinuance of the infusion. If the hypotensive reaction is severe, the use of antihistamines, corticosteroids, or IV fluids may be necessary. The hypotensive reaction is related to the rate of infusion of vancomycin and has been reported most frequently when the drug was administered over a period of 10 minutes or less; however, the reaction may also occur rarely when the drug is infused over a period of 1 hour or longer. To minimize the risk of a hypotensive reaction, vancomycin should be infused over a period of at least 1 hour and the patient's blood pressure should be monitored during the infusion. In patients who have had the reaction, subsequent doses of vancomycin can usually be given without adverse effect if administered at a slow rate (e.g., over several hours). Pretreatment with antihistamines may be of benefit. If attempts to minimize the reaction fail, use of another anti-infective agent may be necessary. The reaction reportedly has occurred in more than 50% of healthy individuals given vancomycin but less frequently when the drug is used therapeutically. Urticaria, exfoliative dermatitis, macular rashes, eosinophilia, vasculitis, a shock-like state, transient anaphylaxis, and, occasionally, vascular collapse have been reported in patients receiving vancomycin. The drug also has been associated with Stevens-Johnson syndrome in at least one patient. A throbbing pain in the muscles of the back and neck has been reported with vancomycin and can usually be minimized or avoided by slower administration of the drug. In patients undergoing continuous ambulatory peritoneal dialysis (CAPD), intraperitoneal administration of vancomycin has been associated with chemical peritonitis, a syndrome consisting of a cloudy dialysate, which may be accompanied by abdominal pain and fever. Chemical peritonitis usually disappears shortly after discontinuance of intraperitoneal vancomycin. Other adverse effects of vancomycin include chills and fever. Priapism after a second IV dose of vancomycin, with recurrence on inadvertent rechallenge, occurred in a 37-year-old man with severe underlying diabetes mellitus; bilateral phlebotomy of the corpus cavernosum resulted in resolution of the priapism. For more Drug Warnings (Complete) data for Vancomycin (22 total), please visit the HSDB record page. Pharmacodynamics Vancomycin is a branched tricyclic glycosylated nonribosomal peptide often reserved as the "drug of last resort", used only after treatment with other antibiotics has failed. Vancomycin has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections: Listeria monocytogenes, Streptococcus pyogenes, Streptococcus pneumoniae (including penicillin-resistant strains), Streptococcus agalactiae, Actinomyces species, and Lactobacillus species. The combination of vancomycin and an aminoglycoside acts synergistically in vitro against many strains of Staphylococcus aureus, Streptococcus bovis, enterococci, and the viridans group streptococci. |
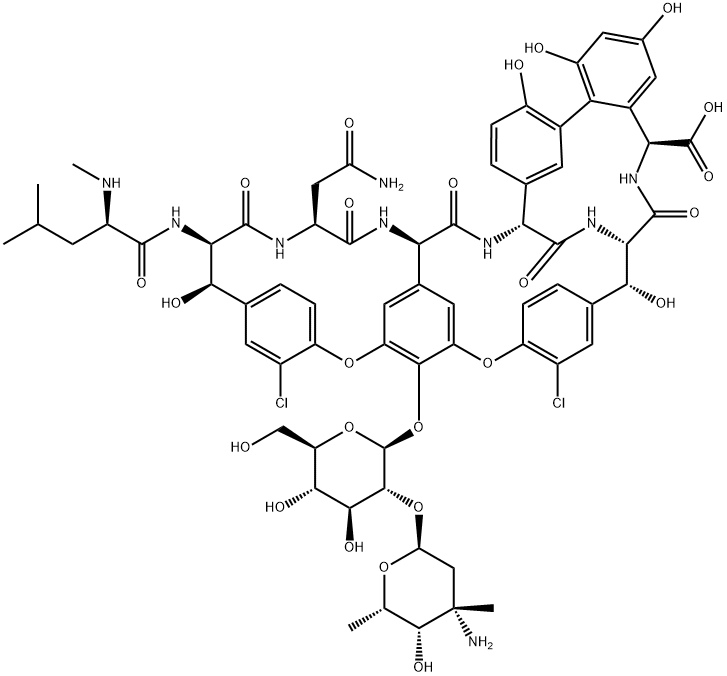
| 分子式 |
C66H75CL2N9O24
|
|---|---|
| 分子量 |
1449.25
|
| 精确质量 |
1447.43
|
| CAS号 |
1404-90-6
|
| 相关CAS号 |
Vancomycin hydrochloride;1404-93-9
|
| PubChem CID |
14969
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.65 g/cm3
|
| LogP |
4.734
|
| tPSA |
530.49
|
| 氢键供体(HBD)数目 |
19
|
| 氢键受体(HBA)数目 |
26
|
| 可旋转键数目(RBC) |
13
|
| 重原子数目 |
101
|
| 分子复杂度/Complexity |
2960
|
| 定义原子立体中心数目 |
18
|
| SMILES |
ClC1C([H])=C2C([H])=C([H])C=1OC1=C([H])C3[C@]([H])(C(N([H])[C@@]4([H])C(N([H])[C@]([H])(C(N([H])[C@]([H])(C(=O)O[H])C5C([H])=C(C([H])=C(C=5C5=C(C([H])=C([H])C4=C5[H])O[H])O[H])O[H])=O)[C@@]([H])(C4C([H])=C([H])C(=C(C=4[H])Cl)OC(C=3[H])=C1O[C@@]1([H])[C@@]([H])([C@]([H])([C@@]([H])([C@@]([H])(C([H])([H])O[H])O1)O[H])O[H])O[C@@]1([H])C([H])([H])[C@@](C([H])([H])[H])([C@@]([H])([C@]([H])(C([H])([H])[H])O1)O[H])N([H])[H])O[H])=O)=O)N([H])C([C@]([H])(C([H])([H])C(N([H])[H])=O)N([H])C([C@@]([H])([C@]2([H])O[H])N([H])C([C@@]([H])(C([H])([H])C([H])(C([H])([H])[H])C([H])([H])[H])N([H])C([H])([H])[H])=O)=O)=O
|
| InChi Key |
MYPYJXKWCTUITO-LYRMYLQWSA-N
|
| InChi Code |
InChI=1S/C66H75Cl2N9O24/c1-23(2)12-34(71-5)58(88)76-49-51(83)26-7-10-38(32(67)14-26)97-40-16-28-17-41(55(40)101-65-56(54(86)53(85)42(22-78)99-65)100-44-21-66(4,70)57(87)24(3)96-44)98-39-11-8-27(15-33(39)68)52(84)50-63(93)75-48(64(94)95)31-18-29(79)19-37(81)45(31)30-13-25(6-9-36(30)80)46(60(90)77-50)74-61(91)47(28)73-59(89)35(20-43(69)82)72-62(49)92/h6-11,13-19,23-24,34-35,42,44,46-54,56-57,65,71,78-81,83-87H,12,20-22,70H2,1-5H3,(H2,69,82)(H,72,92)(H,73,89)(H,74,91)(H,75,93)(H,76,88)(H,77,90)(H,94,95)/t24-,34+,35-,42+,44-,46+,47+,48-,49+,50-,51+,52+,53+,54-,56+,57+,65-,66-/m0/s1
|
| 化学名 |
(1S,2R,18R,19R,22S,25R,28R,40S)-48-[(2S,3R,4S,5S,6R)-3-[(2S,4S,5S,6S)-4-amino-5-hydroxy-4,6-dimethyloxan-2-yl]oxy-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-22-(2-amino-2-oxoethyl)-5,15-dichloro-2,18,32,35,37-pentahydroxy-19-[[(2R)-4-methyl-2-(methylamino)pentanoyl]amino]-20,23,26,42,44-pentaoxo-7,13-dioxa-21,24,27,41,43-pentazaoctacyclo[26.14.2.23,6.214,17.18,12.129,33.010,25.034,39]pentaconta-3,5,8(48),9,11,14,16,29(45),30,32,34(39),35,37,46,49-pentadecaene-40-carboxylic acid
|
| 别名 |
Vancocin;Vancoled; Vancomicina; Vancomycine; Vancomycinum; VANCOR
|
| HS Tariff Code |
3004209090
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~125 mg/mL (~86.25 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 4.17 mg/mL (2.88 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 41.7 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 4.17 mg/mL (2.88 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 41.7 mg/mL 澄清 DMSO 储备液加入 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.6900 mL | 3.4501 mL | 6.9001 mL | |
| 5 mM | 0.1380 mL | 0.6900 mL | 1.3800 mL | |
| 10 mM | 0.0690 mL | 0.3450 mL | 0.6900 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|
|
|
|
|