| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|

| 靶点 |
HSV-1 (IC50 = 2.9 μg/mL)
|
|---|---|
| 体外研究 (In Vitro) |
在浓度分别为 1.64 mM 和 23.34 nmol/mg 蛋白质/5 分钟时,盐酸伐昔洛韦(valaciclovir salted,VACV)的最大摄取速率呈浓度依赖性且可饱和。 VACV 的体外肠道转运特性由 hPEPT1 主导,在 hPEPT1/CHO 细胞、大鼠和兔组织以及 Caco-2 细胞中观察到的非常相似的 Km 值证明了这一点 [5]。
|
| 体内研究 (In Vivo) |
一项重要的比较试验发现,盐酸伐昔洛韦(1 g,每日两次)持续 10 天与阿昔洛韦(200 mg,每日 5 次)治疗首次发作的生殖器疱疹同样有效。两项试验发现,在为期五天的治疗周期中,伐昔洛韦(200 毫克,每日五次)与阿昔洛韦(200 毫克,每日五次)对于控制复发同样有效。伐昔洛韦每天 1 克的剂量与每天 2 克的效果相同。每天可以给予一剂伐昔洛韦[1]。口服伐昔洛韦 1,000 mg,每天 3 次,六天后,在稳态下评估血清和脑脊液阿昔洛韦浓度 [2]。 PE 和 AC 在 3T3 细胞中的 EC50 值分别为 0.02 和 0.01 ug/ml,但在 BHK 细胞中分别为 0.2 和 0.03 ug/ml。用FA和VA治疗感染的免疫抑制小鼠(每天两次,5.5天)以消除耳轻瘫、耳部病变(水泡等)和死亡。红斑比例也从 100% 降低至 24% 和 38%。到第六天,病毒已经从耳朵和脑干消失,但在接受 VA 治疗的小鼠中,当药物停止时,病毒又回来了 [3]。
|
| 酶活实验 |
通过使用斑块减少测定法测定HSV-1W菌株的体外50%抑制浓度(IC50),以验证其对阿昔洛韦的敏感性。HSV-1 W的IC50测定为2.9µg/ml[4]。
|
| 动物实验 |
Forty-seven NZW rabbits latently infected with HSV-1 W strain were divided into four groups: I, 50 mg/kg/day valacyclovir; II, 100 mg/kg/day valacyclovir; III, 150 mg/kg/day valacyclovir; and IV, saline control. One half of the total dose of valacyclovir was delivered via intraperitoneal injections twice daily for 7 days beginning with one dose before excimer laser keratectomy. HSV-1 ocular shedding was determined from eye cultures for 7 days after treatment.The administration of both 100 mg/kg/day (group II) and 150 mg/kg/day (group III) of valacyclovir significantly reduced the number of eyes from which latent HSV-1 was recovered compared with the control group. There was no difference between the control group and group I (50 mg/kg/day valacyclovir). However, all three valacyclovir dosages significantly reduced the total number of HSV-1 shedding days compared with the control group, and 100% HSV-1 TG latency was demonstrated for all four groups.[4]
|
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation The dosage of acyclovir in milk after valacyclovir is less than 1% of a typical infant dosage and would not be expected to cause any adverse effects in breastfed infants. No special precautions are required when using valacyclovir during breastfeeding. In one study, administration of valacyclovir to mothers with concurrent herpes simplex type 2 and HIV infections reduced breastmilk shedding of the HIV virus in breastmilk at 6 and 14 weeks postpartum, but not later.[1] In another study in HIV-positive mothers, valacyclovir did not reduced breastmilk shedding of cytomegalovirus (CMV) or infant CMV acquisition.[2] ◉ Effects in Breastfed Infants In a study of pregnant women with concurrent HIV and Herpes simplex infections, mothers received zidovudine 300 mg daily from week of pregnancy until 12 months postpartum and nevirapine at delivery. Half of the women (n = 74) also received valacyclovir 500 mg orally twice daily from 34 weeks gestation until 12 months postpartum. At 6 weeks postpartum, all infants who received acyclovir in breastmilk had normal serum creatinine (<0.83 mg/dL). Their median serum creatinine and alanine aminotransferase (ALT) values, and growth were no different from those of unexposed infants, with the exception of one infant with an ALT level of 70.1 units/L. Infants whose mothers received valacyclovir generally had adverse effects that were similar to the placebo group, except that treated infants had a lower risk of eczema and oral thrush than infants in the placebo arm.[1][4] ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| 参考文献 |
|
| 其他信息 |
Valacyclovir hydrochloride is an organic molecular entity.
Valacyclovir hydrochloride is an antiviral prescription medicine approved by the U.S. Food and Drug Administration (FDA) to: Treat and/or prevent certain types of herpes simplex virus (HSV) infections, including genital herpes (genital lesions) and cold sores (herpes labialis) Reduce the risk of transmitting genital herpes to other people Treat varicella zoster virus (VZV) infections, including chicken pox (primary varicella infection) and shingles (herpes zoster) HSV and VZV infections can be opportunistic infections (OIs) of HIV. An OI is an infection that occurs more frequently or is more severe in people with weakened immune systems—such as people with HIV—than in people with healthy immune systems. Valacyclovir Hydrochloride is the hydrochloride salt of valacyclovir. Valacyclovir is an acyclovir prodrug that, after metabolization, inhibits viral DNA replication. It is used in the management of herpes simplex and varicella zoster infections, as well as prophylactically for human cytomegalovirus infections. A prodrug of acyclovir that is used in the treatment of HERPES ZOSTER and HERPES SIMPLEX VIRUS INFECTION of the skin and mucous membranes, including GENITAL HERPES. See also: Acyclovir (has active moiety); Valacyclovir (has active moiety). |
| 分子式 |
C13H21CLN6O4
|
|---|---|
| 分子量 |
360.799
|
| 精确质量 |
360.131
|
| 元素分析 |
C, 43.28; H, 5.87; Cl, 9.83; N, 23.29; O, 17.74
|
| CAS号 |
124832-27-5
|
| 相关CAS号 |
Valacyclovir-d8 hydrochloride;1279033-32-7;Valacyclovir;124832-26-4;Valacyclovir-d4 hydrochloride;1331910-75-8;Valacyclovir hydrochloride hydrate;1218948-84-5; 124832-27-5 (HCl); 950189-66-9
|
| PubChem CID |
135398741
|
| 外观&性状 |
Typically exists as white to off-white solids at room temperature
|
| 密度 |
1.55g/cm3
|
| 沸点 |
588.4ºC at 760 mmHg
|
| 熔点 |
170-172ºC
|
| 闪点 |
309.7ºC
|
| 蒸汽压 |
7.95E-14mmHg at 25°C
|
| 折射率 |
1.673
|
| LogP |
1.285
|
| tPSA |
151.14
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
483
|
| 定义原子立体中心数目 |
1
|
| SMILES |
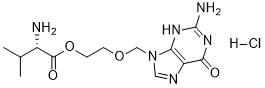
N[C@@H](C(C)C)C(OCCOCN1C=NC2=C1N=C(N)NC2=O)=O.Cl
|
| InChi Key |
ZCDDBUOENGJMLV-QRPNPIFTSA-N
|
| InChi Code |
InChI=1S/C13H20N6O4.ClH/c1-7(2)8(14)12(21)23-4-3-22-6-19-5-16-9-10(19)17-13(15)18-11(9)20;/h5,7-8H,3-4,6,14H2,1-2H3,(H3,15,17,18,20);1H/t8-;/m0./s1
|
| 化学名 |
2-[(2-amino-6-oxo-1H-purin-9-yl)methoxy]ethyl (2S)-2-amino-3-methylbutanoate;hydrochloride
|
| 别名 |
Zelitrex; 256U87; Vacyclovir; 256U; L-valyl ester; BW256U87; Valacyclovir Hydrochloride; Valtrex L-valylacyclovir; Valacyclovir HCl; Valtrex; Valaciclovir hydrochloride; Valaciclovir Hcl; 256U87 hydrochloride; BW-256U87;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ~100 mg/mL (~277.16 mM)
DMSO : ~43.33 mg/mL (~120.09 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.93 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.93 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.93 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 100 mg/mL (277.16 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7716 mL | 13.8581 mL | 27.7162 mL | |
| 5 mM | 0.5543 mL | 2.7716 mL | 5.5432 mL | |
| 10 mM | 0.2772 mL | 1.3858 mL | 2.7716 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Randomized open label clinical trial directed to optimize the duration of empirical antimicrobial therapy in haematologic patients with febrile neutropenia
CTID: null
Phase: Phase 3 Status: Completed
Date: 2012-03-26
|