| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
HIV; non-nucleoside reverse transcriptase
|
|---|---|
| 体外研究 (In Vitro) |
MK-8507是一种新型的HIV-1非核苷逆转录酶抑制剂,正在开发用于治疗HIV-1感染。MK-8507在体外具有很高的抗病毒效力和药代动力学(PK)特性,支持每周给药一次[1]。
|
| 体内研究 (In Vivo) |
共招募了18名参与者(每组6名)。在评估的剂量范围内,给药后7天HIV-1 RNA的平均减少量为1.2至1.5 log10拷贝/mL。一名患者在给药后14天出现与F227C逆转录酶变体(每链终止法测序)相关的病毒反弹;通过超深度测序在另一名参与者身上发现了这种变异,作为一种新兴的少数变异。MK-8507 PKs通常与剂量成正比,与先前研究中未感染HIV-1的参与者的观察结果相似;本研究中MK-8507的平均半衰期为56-69小时。MK-8507在所有剂量下均具有良好的耐受性[1]。
|
| 动物实验 |
In 3 sequential panels, participants aged 18-60 years with baseline plasma HIV-1 RNA ≥10,000 copies/mL and CD4+ T-cell count >200/mm3 received a single oral dose of 40, 80, or 600 mg MK-8507 in the fasted state. Participants were assessed for HIV-1 RNA for at least 7 days, PKs for 14 days, and safety and tolerability for 21 days postdose.[1]
Pharmacokinetics[1] MK-8507 in plasma was extracted by protein precipitation and analyzed by Merck & Co., Inc., (West Point, PA) using liquid–liquid extraction for analyte isolation followed by liquid chromatographic-tandem mass spectrometric detection. The lower limit of quantitation was 1.0 ng/mL (2.03 nM) with a linear calibration range from 1.0 to 1000 ng/mL. All PK parameter values were calculated by noncompartmental analysis using the software Phoenix WinNonlin Professional (Version 6.3; Certara, Princeton, NJ). Maximum plasma concentration (Cmax), plasma concentration at 168 hours (C168hr), and time to maximum plasma concentration (Tmax) were generated from the observed plasma concentration time data. Area under the concentration–time curve from time 0 to infinity (AUC0–∞) and time 0–168 hours (AUC0–168) were calculated using the linear trapezoidal method for ascending concentrations and the log trapezoidal method for descending concentrations (linear-up/log-down). The PK/pharmacodynamic (PD) relationship was assessed by examining the correlation between MK-8507 C168hr and the reduction in plasma HIV-1 RNA levels from baseline at 168 hours (7 days) postdose with a nonlinear least squares approach using an Emax model. The nonlinear least squares analysis was conducted using R (version 3.6.3; R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/). |
| 药代性质 (ADME/PK) |
Plasma MK-8507 PKs are summarized in Table Table44 and plasma concentration–time profiles are shown in Figure Figure1B.1B. MK-8507 PKs were generally dose-proportional. The mean terminal plasma half-life of MK-8507 ranged from 56 to 69 hours across dose groups.[1]
A scatterplot of individual weekly Ctrough (C168hr) and HIV-1 RNA change from baseline at 7 days is shown in Figure Figure1C.1C. In general, most data points seem to be on the flat, maximal-effect portion of the exposure–response relationship, with a trend toward decreased response at the lower end of the C168hr range at the 40 mg dose.[1] |
| 毒性/毒理 (Toxicokinetics/TK) |
MK-8507 was generally well tolerated and no participant discontinued the study because of an AE. Seven participants experienced a total of 10 AEs. The most common were nasopharyngitis (n = 3, 2 moderate and 1 mild) and headache (n = 3, 2 moderate and 1 mild). Of the 10 AEs, only the 3 events of headache were considered by the investigator to be related to the study drug. There were no clinically meaningful trends in vital signs, electrocardiograms, or laboratory tests. One participant who received 600 mg MK-8507 was diagnosed with diffuse large B cell lymphoma after having started SOC ART; this serious AE was not considered related to study drug and the participant recovered with sequelae. All other AEs were mild or moderate in intensity and resolved by the end of the study.[1]
|
| 参考文献 | |
| 其他信息 |
Conclusions: The robust antiviral activity, PK, and tolerability of MK-8507 support its continued development as part of a complete once weekly oral regimen for HIV-1 treatment; combination therapy could mitigate the emergence of resistance-associated variants.[1]
|
| 分子式 |
C18H8CLF6N5O3
|
|---|---|
| 分子量 |
491.731243133545
|
| 精确质量 |
491.021
|
| 元素分析 |
C, 43.97; H, 1.64; Cl, 7.21; F, 23.18; N, 14.24; O, 9.76
|
| CAS号 |
1591823-76-5
|
| PubChem CID |
73505111
|
| 外观&性状 |
White to light yellow solid powder
|
| LogP |
2.7
|
| tPSA |
107
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
12
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
33
|
| 分子复杂度/Complexity |
1020
|
| 定义原子立体中心数目 |
0
|
| SMILES |
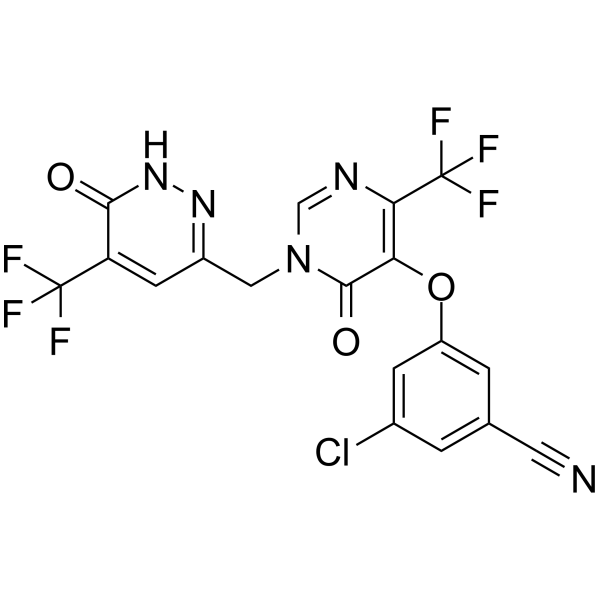
C(#N)C1=CC(OC2C(=O)N(CC3=NNC(=O)C(C(F)(F)F)=C3)C=NC=2C(F)(F)F)=CC(Cl)=C1
|
| InChi Key |
YSFHLBYWQCLYIY-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C18H8ClF6N5O3/c19-9-1-8(5-26)2-11(3-9)33-13-14(18(23,24)25)27-7-30(16(13)32)6-10-4-12(17(20,21)22)15(31)29-28-10/h1-4,7H,6H2,(H,29,31)
|
| 化学名 |
3-chloro-5-{[6-oxo-1-{[6-oxo-5-(trifluoromethyl)-1,6- dihydropyridazin-3-yl]methyl}-4-(trifluoromethyl)-1,6- dihydropyrimidin-5-yl]oxy}benzonitrile
|
| 别名 |
MK8507; MK-8507; Ulonivirine; 1591823-76-5; 4NS011EGKZ; 3-chloro-5-((6-oxo-1-((6-oxo-5-(trifluoromethyl)-1,6-dihydropyridazin-3-yl)methyl)-4-(trifluoromethyl)-1,6-dihydropyrimidin-5-yl)oxy)benzonitrile; UNII-4NS011EGKZ; MK8507; WHO 12037; MK 8507
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~250 mg/mL (~508.41 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.08 mg/mL (4.23 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.23 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0336 mL | 10.1682 mL | 20.3364 mL | |
| 5 mM | 0.4067 mL | 2.0336 mL | 4.0673 mL | |
| 10 mM | 0.2034 mL | 1.0168 mL | 2.0336 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。