| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
在重塑人类皮肤 (RHE) 过程中,trifarotene (CD5789)(3.3 μL 0.33 cm2;24 小时)参与角化、脱屑、表皮化和细胞标记。 trifarotene 所有组合靶基因的平均 EC50 为 0.0048% [2]。
|
|---|---|
| 体内研究 (In Vivo) |
已经证明,当以 0.01% 的致粉刺剂量给药时,曲法罗汀(乳膏中 0.001%-0.01%,每只小鼠 25 毫克)完全有效(减少 98%)[2]。
|
| 动物实验 |
Animal/Disease Models: Rhino mouse[2]
Doses: 0.001%, 0.0025%, 0.005% and 0.01% cream, the dose is 25 mg/mouse (5 cm2 surface of back skin, calculated as 5 mg/cm2) one time/day; 11 days Experimental Results: Epidermal thickness increased by 275% (66 μm) and transepidermal water loss (TEWL) increased by 285% (26 g/h/m2). |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Systemic absorption of trifarotene is minimal. In a pharmacokinetic study involving 19 subjects, systemic concentrations were only quantifiable in 7 - steady state Cmax values ranged from undetectable (<5 pg/mL) to 10 pg/mL and AUC0-24h ranged from 75 to 104 pg.h/mL. Trifarotene is eliminated primarily in the feces. Metabolism / Metabolites Trifarotene is rapidly metabolized in human hepatocytes - its observed half-life in human keratinocytes is >24 hours, whereas half-life in human liver microsomes is approximately 5 minutes. Metabolism of trifarotene is catalyzed primarily by CYP2C9, CYP3A4, CYP2C8, and, to a lesser extent, CYP2B6. Biological Half-Life The terminal half-life of trifarotene is typically between 2 to 9 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Trifarotene has not been studied during breastfeeding. Because it is poorly absorbed after topical application, it is a low risk to the nursing infant. Do not apply trifarotene cream directly to the nipple and areola and ensure that the infant's skin does not come into direct contact with the areas of skin that have been treated. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Trifarotene is 99.9% protein bound in plasma. |
| 参考文献 |
|
| 其他信息 |
Trifarotene is a topical retinoid cream used in the treatment of acne vulgaris that was first approved for use in the United States in October 2019. Retinoids are a class of medications structurally and functionally analogous to [vitamin A], though later generation retinoids such as trifarotene and [adapalene] bear little structural resemblance to vitamin A and are analogous only in function. Trifarotene is considered the first of the "fourth-generation" retinoids due to its uniquely selective activity - this selectivity appears to confer improved efficacy and reduced side effects as compared to older, less selective retinoids.
Trifarotene is a Retinoid. Trifarotene is a selective retinoic acid receptor gamma (RAR gamma) agonist that can be used in the treatment of acne vulgaris. Upon topical application, trifarotene selectively binds to the RAR gamma receptor, thereby altering the expression of certain genes that are involved in inflammation and cellular differentiation. Drug Indication Trifarotene is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older. Treatment of ichthyoses Treatment of acne Mechanism of Action Trifarotene is a potent and selective agonist of retinoic acid receptor-γ (RAR-γ). It has significantly less activity at RAR-β and RAR-α (16- and 65-fold lower than activity at RAR-γ, respectively), and has no activity at retinoid X receptors (RXRs). Agonism at retinoic acid receptors results in dimerization, and the resulting receptor-ligand dimer binds to specific DNA regulatory sequences (retinoic acid response elements, or RAREs) in the promotor regions of retinoid-responsible genes. Downstream alterations to gene expression induced by binding to these regions is the principle mechanism through which trifarotene exerts its comedolytic, anti-inflammatory, and depigmenting effects. Like other retinoids, trifarotene influences the expression of a number of genes involved in retinoid metabolism, epidermal differentiation/proliferation, and epidermal response to stress. In addition, trifarotene appears to modulate retinoid-mediated pathways involved in proteolysis, skin hydration, and cell adhesion - modulation of these additional pathways has not been observed with other retinoids and may therefore be unique to trifarotene. Pharmacodynamics Trifarotene exerts its effects via agonism at retinoid receptors - these receptors function to alter DNA transcription, resulting in downstream modulation of the expression of various genes involved in acne pathogenesis. It may be associated with skin irritation and should not be applied to cuts, abrasions, or otherwise damaged skin. As trifarotene may result in photosensitivity, patients should be cautioned to avoid excess sun exposure and to use sunscreen and/or protective clothing if exposure is unavoidable. |
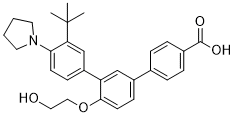
| 分子式 |
C29H33NO4
|
|---|---|
| 分子量 |
459.586
|
| 精确质量 |
459.24
|
| CAS号 |
895542-09-3
|
| 相关CAS号 |
895542-09-3;
|
| PubChem CID |
11518241
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
641.9±55.0 °C at 760 mmHg
|
| 熔点 |
245C
|
| 闪点 |
342.0±31.5 °C
|
| 蒸汽压 |
0.0±2.0 mmHg at 25°C
|
| 折射率 |
1.599
|
| LogP |
6.27
|
| tPSA |
70
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
34
|
| 分子复杂度/Complexity |
647
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
MFBCDACCJCDGBA-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C29H33NO4/c1-29(2,3)25-19-23(10-12-26(25)30-14-4-5-15-30)24-18-22(11-13-27(24)34-17-16-31)20-6-8-21(9-7-20)28(32)33/h6-13,18-19,31H,4-5,14-17H2,1-3H3,(H,32,33)
|
| 化学名 |
3''-(tert-butyl)-4'-(2-hydroxyethoxy)-4''-(pyrrolidin-1-yl)-[1,1'
|
| 别名 |
CD5789 CD-5789 CD 5789
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~250 mg/mL (~543.97 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.53 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.53 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1759 mL | 10.8793 mL | 21.7585 mL | |
| 5 mM | 0.4352 mL | 2.1759 mL | 4.3517 mL | |
| 10 mM | 0.2176 mL | 1.0879 mL | 2.1759 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。