| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
BMX (IC50 = 6 nM); BTK (IC50 = 6.8 nM); TEC (IC50 = 48 nM); TXK (IC50 = 92 nM); BLK (IC50 = 0.3 μM); ERBB4 (IC50 = 0.77 μM); EGFR (IC50 = 3.02 μM); JAK3 (IC50 = 5.52 μM); ERBB2 (IC50 = 7.31 μM)
|
|---|---|
| 体外研究 (In Vitro) |
Tirabrutinib(0.1-1000 nM 或 0.001-100 nM;72 h)的 IC50 值分别为 9.127 nM 和 17.10 nM,限制 OCI-L Y10 和 SU-DHL-6 细胞的生长[1]。
Tirabrutinib(0.5、5、50 μM;24、48 小时)诱导 SU-DHL-6 细胞凋亡;然而,它需要高剂量和长时间给药(在高达 50 μM 的浓度下孵育 48 小时)[1]。 Tirabrutinib(300 nM,72 小时)会导致 TMD8 细胞中 caspase-3 和 PARP 裂解[ 2]。 Tirabrutinib与BTK Cys-481不可逆共价结合。测量了失活效率kinact/Ki,并用于计算所研究的四种抑制剂在不同激酶之间的选择性。Tirabrutinib对BTK的kinact/Ki值为2.4±0.6 × 104 M-1 s-1,对重要脱靶有选择性。 结论:对于本研究中测试的BTK抑制剂,失活动力学分析比传统的单时间点抑制测量更准确地测量了效力和选择性。临床测试的BTK抑制剂之间存在细微但明显的差异,这可能转化为临床疗效和安全性的差异。 一般意义:这是第一个提供四种临床相关BTK抑制剂关于BTK和相关激酶失活的详细并排比较的研究。[3] |
| 体内研究 (In Vivo) |
替拉替尼(10 mg/kg;口服;单次)快速进入大脑和血浆,给药后两小时达到其 Cmax(血液 Cmax = 339.53 ng/mL,脑 Cmax = 28.9 ng/mL)[1].
替拉替尼(6、20 mg/kg;口服;每日一次,持续 3 周)可抑制体内肿瘤生长[2] 。 |
| 酶活实验 |
共价结合的测定[3]
蛋白标记实验采用终浓度为2 μM的BTK,在含有10 mM HEPES、pH 7.5、150 mM氯化钠、10 mM氯化镁、2 mM Tris(2-羧乙基)膦(TCEP)和1%甘油的缓冲溶液中进行。抑制剂的终浓度为10 μM,所有样品的终浓度为1% DMSO。四种条件分别为:BTK +替拉替尼、BTK + staurosporine、BTK +依鲁替尼、BTK + DMSO对照。添加化合物后,样品在旋转摇床(1200 rpm)中于4°C下孵育过夜。孵育18小时后,从每种条件中收集等分进行分析,此时间点称为t =预孵育。然后对剩余样品进行后续处理,将伊鲁替尼加入BTK +替若替尼和BTK + staurosporine样品中,最终浓度为100 μM。在BTK +依鲁替尼和BTK + DMSO对照样品中加入等量的DMSO以保持相同的体积。在4℃下孵育6小时后,在最终时间点(t = post-chase)收集剩余样品。在两个时间点采集的等分在收集时使用质谱法和酶活性测定法进行分析。 质谱分析采用Agilent 6210飞行时间质谱仪和Agilent 1200快速分辨率高效液相色谱,使用Masshunter B.05采集软件。样品在Agilent Zorbax 300 Extend C18快速分辨柱上运行,温度为70°C,采用反相色谱法,梯度从20%到90%乙腈(含0.1%甲酸)。使用Agilent MassHunter Qualitative Analysis B.06处理数据,采用BioConfirm工作流,允许蛋白质反卷积以获得中性质量值。 BTK Cys-481的共价结合[3] 样品制备方法为:25 μg (2 μM)的内部重组BTK蛋白在50 mM碳酸氢铵和10 μM替拉替尼中37℃孵育1 h。然后用5 mM DTT在55°C下还原45分钟,然后用10 mM碘乙酰胺在25°C下烷基化1小时。然后用50:1的葡聚糖内源性蛋白酶在37°C下酶切12小时,然后以50:1的比例加入胰蛋白酶,再在37°C下酶切4小时,得到序列为YMANGCLLNYLR的BTK的预期肽目标。消化后的样品冻干,再悬浮在3%乙腈、0.1%甲酸中,进行质谱分析。 质谱分析方法如下:样品使用ThermoFisher UltiMate 3000 rslnano系统进样。分离采用ThermoFisher Scientific ES800 Easy Spray LC色谱柱(150 mm × 75 μm),流速为300 nL/min,梯度为60 min,使用1%乙腈,0.1%甲酸为溶剂a, 90%乙腈,0.1%甲酸为溶剂B [3% B - 35% B (45 min), 35% B - 90% B (15 min), 90% B (5 min),再平衡(20 min)]。质谱分析在ThermoFisher Q-Exactive HF上进行,使用前20个数据依赖采集。自动增益控制设置使用50 ms填充时间和3E6离子计数的ms扫描(60 K分辨率)和100 ms文件时间和1E5离子计数的MSMS扫描(15 K分辨率)。使用Proteome Discoverer 2.2对Swissprot人类数据库进行数据检索,使用tirabrutinib进行可变化学修饰。 View More
Z'-LYTE™和LanthaScreen™试验中tirabrutinib、ibrutinib、acalabrutinib和spebrutinib对BTK和其他酪氨酸激酶的IC50测定[3] BTK酶活性与IC50评价[3] 通过使用LanthaScreen™Assay Kit测定荧光素标记底物的磷酸化来定量BTK活性。最终的反应混合物含有激酶缓冲液A [50 mM HEPES (pH 7.5), 10 mM MgCl2, 0.01% brij-35, 1 mM EGTA和0.5 mg/mL BSA], 200-300 pM BTK, 0.2 μM荧光素- poly GT底物和180 μM ATP (2× Km)。所有预孵育和反应均在黑色96孔非结合表面(NBS™)分析板中进行,室温下进行。为了评估质谱分析中所用样品的酶活性,将每个样品的等分液稀释至300 pM BTK和1.5 nM激酶反应抑制剂. |
| 细胞实验 |
细胞系:SU-DHL-6 和 OCI-L Y10 细胞
浓度:0.1-1000 nM; 0.001 nM-100 nM 孵育时间:72 h 结果:对 OCI-L Y10 和 SU-DHL-6 细胞具有良好的抗增殖活性,IC50 分别为 9.127 nM 和 17.10 nM。 细胞死亡评估[2] 本研究中使用的细胞系以前已经描述过,并且是从发起人或从Deutsche Sammlung von microorganismen und Zellkulturen GmbH (DSMZ)获得的。通过中期细胞遗传学和短串联重复评估证实了细胞系的身份。细胞系在添加10%胎牛血清的RPMI 1640培养基中生长。细胞系对Tirabrutinib的敏感性以及联合处理使用CellTiterGlo®活力测定或AnnexinV-FITC染色进行。EC50的计算使用GraphPadPrism完成。Tirabrutinib耐药TMD8 (TMD8R)是通过连续暴露在3 nM至1000 nM的Tirabrutinib浓度下超过9个月产生的,直到对Tirabrutinib建立稳定的耐药性。其中,从3、6、12、25、40、60、80、100、200、400、800、1000 nM的浓度逐渐增加。细胞传代每周2次。当细胞生长良好时,用含有相同浓度的替拉替尼的新鲜培养基重新悬浮细胞(最终细胞密度为100,000个细胞/mL)。细胞对替拉替尼的敏感性在每一步通过CellTiterGlo®检测。通过Sanger测序确定TMD8R的突变状态。根据秋-塔拉莱的多重药效方程,用CalcuSyn计算联合指数。 |
| 动物实验 |
Male SD rats (219.0–260.5g)
10 mg/kg Oral administration; single. Mouse Xenograft Model[2] To assess in vivo efficacy of tirabrutinib, severe combined immunodeficiency (SCID) mice were injected with 1 × 107 cells in Matrigel, subcutaneously. Randomization and treatment were initiated when the mean tumor volumes reached 400 mm3 for TMD8 and 200 mm3 for tirabrutinib resistant cells. Groups of mice were then dosed via diet containing tirabrutinib at concentrations of 0.0037%, 0.012% and 0.037%. The daily dosage was found to be comparable to the doses, 6, 20, and 60 mg/kg/day, respectively. Tumor growth was assessed using a caliper. |
| 药代性质 (ADME/PK) |
Ibrutinib and Tirabrutinib might be more suitable for brain-confined diseases than zanubrutinib[1]
As indicated by the inhibition and apoptosis assay, high level of drug concentration in a prolonged period is required for the complete inhibition of tumor cells. Therefore, to determine the feasibility of BTK inhibitors in treating PCNSL, we tested if they were able to maintain the concentration in brain efficient for tumor inhibition. SD rats were orally administered with BTK inhibitors once. Plasma and brain tissue were sampled at designated time points to test the drug concentration (Figure 4). As indicated by Figure 4, all three BTK inhibitors were rapidly absorbed into plasma and brain. Both unbound ibrutinib and Tirabrutinib reached Cmax 2 hours post administration, and maintained at a relatively stable level, both in blood and brain tissue (ibrutinib: blood Cmax =412.7 ng/mL, brain Cmax =40.4 ng/mL, n=3; Tirabrutinib: blood Cmax =339.53 ng/mL, brain Cmax =28.9 ng/mL, n=3). As for unbound zanubrutinib, it rapidly reached maximum concentration in blood and brain at 0.5-hour post administration, and exhibited a decrease in blood concentration slightly faster than the other BTK inhibitors. In brain, on the other hand, unbound zanubrutinib concentration slumped, and was below the limit of detection 4 hours after administration. Because Cmax in blood and brain were simultaneously reached by each BTK inhibitor (ibrutinib and Tirabrutinib: 2 hour post oral administration; zanubrutinib: 0.5 hour post oral administration), unbound brain-to-plasma concentration ratio was calculated at the time they reached Cmax. Unbound brain-to-plasma concentration ratio of zanubrutinib, Tirabrutinib and ibrutinib were 3.5%, 8.5% and 9.8%, respectively (Table 2). This ratio of ibrutinib is slightly higher than Tirabrutinib. This ratio of zanubrutinib, however, was much lower than ibrutinib and Tirabrutinib, indicating its inferior ability to pass through BBB compared to ibrutinib and Tirabrutinib. Together, these data indicate that ibrutinib, Tirabrutinib and zanubrutinib can be rapidly absorbed into blood and distributed into brain after oral administration. Compared with zanubrutinib, ibrutinib and Tirabrutinib exerted better ability in passing through BBB and maintaining a high and stable concentration in brain, facilitating these inhibitors to exert their anti-tumoral effect in brain, making them more promising candidates for the treatment of PCNSL. |
| 毒性/毒理 (Toxicokinetics/TK) |
ONO-4059 was found to be well tolerated, with no dose limiting toxicities (DLTs). A total of 18 ONO-4059-related adverse events were reported in 6 out of 14 patients; CTCAE-V4.0 G1 (n=10 [n=6 in 1 patient]) and G2 (n=5). Three ONO-4059-related G3 haematological toxicities were reported in 2 patients; thrombocytopenia (x2) and anemia. No ONO-4059-related G4 events, or related SAEs or infections were reported. The pharmacokinetics of ONO-4059 reflects rapid absorption and elimination, a half-life of ∼6 hours, a dose dependent increase in exposure with no accumulation of ONO-4059 exposure and low inter- or intra-patient variability; with Btk occupancy in peripheral blood (as measured by phosphorylated Btk) being maintained for at least 24 hours across all dose levels.
|
| 参考文献 | |
| 其他信息 |
Tirabrutinib is under investigation in clinical trial NCT02626026 (Safety and Pharmacokinetics of GS-4059 in Healthy Volunteers and Subjects With Rheumatoid Arthritis (RA)).
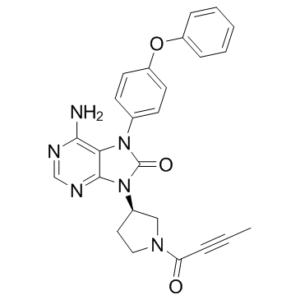
Tirabrutinib is an orally available formulation containing an inhibitor of Bruton agammaglobulinemia tyrosine kinase (BTK), with potential antineoplastic activity. Upon administration, tirabrutinib covalently binds to BTK within B cells, thereby preventing B cell receptor signaling and impeding B cell development. As a result, this agent may inhibit the proliferation of B cell malignancies. BTK, a cytoplasmic tyrosine kinase and member of the Tec family of kinases, plays an important role in B lymphocyte development, activation, signaling, proliferation and survival. |
| 分子式 |
C25H22N6O3
|
|---|---|
| 分子量 |
454.490
|
| 精确质量 |
454.175
|
| 元素分析 |
C, 66.07; H, 4.88; N, 18.49; O, 10.56
|
| CAS号 |
1351636-18-4
|
| 相关CAS号 |
Tirabrutinib hydrochloride;1439901-97-9;ONO-4059 analog;1351635-67-0
|
| PubChem CID |
54755438
|
| 外观&性状 |
White to off white powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
672.0±65.0 °C at 760 mmHg
|
| 闪点 |
360.2±34.3 °C
|
| 蒸汽压 |
0.0±2.1 mmHg at 25°C
|
| 折射率 |
1.700
|
| LogP |
2.31
|
| tPSA |
105Ų
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
34
|
| 分子复杂度/Complexity |
825
|
| 定义原子立体中心数目 |
1
|
| SMILES |
O=C1N(C2C=CC(=CC=2)OC2C=CC=CC=2)C2=C(N)N=CN=C2N1C1CN(C(C#CC)=O)CC1
|
| InChi Key |
SEJLPXCPMNSRAM-GOSISDBHSA-N
|
| InChi Code |
InChI=1S/C25H22N6O3/c1-2-6-21(32)29-14-13-18(15-29)31-24-22(23(26)27-16-28-24)30(25(31)33)17-9-11-20(12-10-17)34-19-7-4-3-5-8-19/h3-5,7-12,16,18H,13-15H2,1H3,(H2,26,27,28)/t18-/m1/s1
|
| 化学名 |
6-amino-9-[(3R)-1-but-2-ynoylpyrrolidin-3-yl]-7-(4-phenoxyphenyl)purin-8-one
|
| 别名 |
ONO-4059; GS4059; ONO-WG-307; ONO4059; GS-4059;ONO 4059; GS-4059; Btk Kinase inhibitor; ONO-4059(Free base); Tirabrutinib [INN]; Tirabrutinib free base; ONO-4059; GS 4059; ONO WG-307
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ≥ 100 mg/mL (~220.0 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.50 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.50 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.50 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2003 mL | 11.0013 mL | 22.0027 mL | |
| 5 mM | 0.4401 mL | 2.2003 mL | 4.4005 mL | |
| 10 mM | 0.2200 mL | 1.1001 mL | 2.2003 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02457598 | Active Recruiting |
Drug: Tirabrutinib Drug: Idelalisib |
B-cell Malignancies | Gilead Sciences | June 16, 2015 | Phase 1 |
| NCT04947319 | Recruiting | Drug: Tirabrutinib | Refractory Primary Central Nervous System Lymphoma Primary CNS Lymphoma |
Ono Pharmaceutical Co. Ltd | December 29, 2021 | Phase 2 |
| NCT02983617 | Completed | Drug: Tirabrutinib Drug: Entospletinib |
Chronic Lymphocytic Leukemia | Gilead Sciences | April 6, 2017 | Phase 2 |
| NCT02968563 | Completed | Drug: Tirabrutinib Drug: Idelalisib |
Chronic Lymphocytic Leukemia | Gilead Sciences | December 13, 2016 | Phase 2 |
| NCT02626026 | Completed | Drug: Tirabrutinib Drug: Placebo |
Rheumatoid Arthritis | Gilead Sciences | January 26, 2016 | Phase 1 |