| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
AMPA receptor
|
|---|---|
| 体外研究 (In Vitro) |
Sunifiram 是一种新型吡咯烷酮益智药,其结构与吡拉西坦类似,后者是为治疗阿尔茨海默病等神经退行性疾病而开发的。为了解答 sunifiram 是否影响海马 CA1 区 N-甲基-D-天冬氨酸受体 (NMDAR) 依赖性突触功能的问题,我们通过电生理学评估了 sunifiram 对 NMDAR 依赖性长期增强 (LTP) 的影响,并通过免疫印迹分析评估了 sunifiram 对突触蛋白磷酸化的影响。在小鼠海马切片中,10-100 nM 的 sunifiram 以钟形剂量反应关系显著增强了 LTP,在 10 nM 时达到峰值。Sunifiram 在 1-1000 nM 的治疗以剂量依赖性方式增加了场兴奋性突触后电位 (fEPSP) 的斜率。这种增强与通过激活 CaMKII 导致的 AMPAR 受体磷酸化增加有关。有趣的是,在基础条件下,sunifiram 处理增加了 PKCα(Ser-657)和 Src 家族(Tyr-416)的活性,其钟形剂量反应曲线与 LTP 的峰值相同,在 10 nM 时达到峰值。7-ClKN 处理可抑制 sunifiram 诱导的 PKCα(Ser-657)和 Src(Tyr-416)磷酸化的增加。sunifiram 引起的 LTP 增强作用被 Src 家族抑制剂 PP2 显著抑制。最后,当用高浓度甘氨酸(300 μM)预处理时,sunifiram 处理无法增强 CA1 区的 LTP。总之,sunifiram 刺激 NMDAR 的甘氨酸结合位点,同时通过 Src 激酶激活 PKCα。 PKCα活性增强会通过激活CaMKII来增强海马LTP。[2]
|
| 体内研究 (In Vivo) |
OBX 小鼠术后 10 天起每天给药一次 sunifiram (0.01-1.0 mg/kg po),连续 7-12 天,联合或不联合使用加维斯汀 (10 mg/kg ip),加维斯汀是 N-甲基-d-天冬氨酸受体 (NMDAR) 的甘氨酸结合位点抑制剂。在 OBX 小鼠中,sunifiram 治疗显著改善了 Y 型迷宫评估的空间参考记忆和新物体识别任务评估的短期记忆。Sunifiram 还恢复了未用加维斯汀治疗的 OBX 小鼠海马 LTP 损伤。相反,sunifiram 治疗并不能改善 OBX 小鼠悬尾任务评估的抑郁行为。值得注意的是,sunifiram 治疗将 OBX 小鼠海马 CA1 区的 CaMKIIα (Thr-286) 自身磷酸化和 GluR1 (Ser-831) 磷酸化恢复到对照小鼠的水平。同样,sunifiram 治疗将 PKCα (Ser-657) 自身磷酸化和 NR1 (Ser-896) 磷酸化改善至对照水平。sunifiram 对 CaMKII 和 PKC 自身磷酸化的刺激通过预先用 gavestinel 治疗而显著抑制。然而,sunifiram 治疗不影响 CaMKIV (Thr-196) 和 ERK 的磷酸化。总之,sunifiram 通过刺激 NMDAR 的甘氨酸结合位点,改善 OBX 引起的记忆相关行为缺陷和海马 CA1 区受损的 LTP。[3]
DM 232和DM 235/sunifiram是结构上与ampakines相关的新型抗记忆化合物。因此,在体内和体外研究了AMPA受体在DM 232和DM 235作用机制中的作用。在小鼠被动回避试验中,这两种化合物(0.1mg/kg(-1)i.p.)都能够逆转AMPA受体拮抗剂NBQX(30mg/kg(-1,i.p.))诱导的失忆。在有效剂量下,所研究的化合物没有损害运动协调性,如旋转棒试验所示,也没有改变自发运动和检查活动,如孔板试验所示。DM 232和DM 235在大鼠海马切片中进行的犬尿酸试验中逆转了犬尿酸诱导的NMDA介导的[(3)H]NA释放的拮抗作用。NBQX取消了这一影响。DM 232在体外以浓度依赖的方式增加大鼠海马中的兴奋性突触传递。这些结果表明,DM 232和DM 235通过激活AMPA介导的神经传递系统充当认知增强剂。[1] 阿尔茨海默病(AD)表现为内侧隔膜胆碱能系统的退化,从而导致患者嗅觉功能下调。我们之前报道过,嗅球切除(OBX)小鼠表现出海马依赖性记忆障碍,这是通过记忆相关行为任务和海马长时程增强(LTP)来评估的。在本研究中,我们重点研究了新型吡咯烷酮促智药物桑尼菲拉姆是否能改善OBX小鼠的记忆障碍和抑郁。从术后10天开始,OBX小鼠每天服用一次桑尼菲拉姆(0.01-1.0mg/kg p.o.),持续7-12天,服用或不服用强饲试验(10mg/kg i.p.),强饲试验是N-甲基-d-天冬氨酸受体(NMDAR)的甘氨酸结合位点抑制剂。通过Y迷宫评估的空间参考记忆和通过新物体识别任务评估的短期记忆在OBX小鼠中通过桑尼菲拉姆治疗得到了显著改善。Sunifiram还恢复了未经强饲试验治疗的OBX小鼠海马LTP损伤。相比之下,桑尼菲拉姆治疗并没有改善OBX小鼠尾部悬吊任务评估的抑郁行为。值得注意的是,桑尼菲拉姆治疗使OBX小鼠海马CA1区的CaMKIIα(Thr-286)自磷酸化和GluR1(Ser-831)磷酸化恢复到对照组小鼠的水平。同样,桑尼菲拉姆治疗将PKCα(Ser-657)自磷酸化和NR1(Ser-896)磷酸化提高到对照水平。灌胃试验预处理可显著抑制桑尼菲拉姆对CaMKII和PKC自磷酸化的刺激。然而,桑尼菲拉姆治疗不影响CaMKIV(Thr-196)和ERK的磷酸化。综上所述,桑尼菲拉姆通过刺激NMDAR的甘氨酸结合位点,改善了OBX诱导的记忆相关行为缺陷和海马CA1区LTP受损[3]。 |
| 酶活实验 |
生化分析[2]
如前所述进行生化分析(Laemmli,1970;Moriguchi等人,2008)。我们使用了如下所列的抗体:抗磷酸化CaMKII(1:5000;Fukunaga等人,2002),抗CaMKII,(1:5000,Fukunaga等,1995),抗磷酸化PKCα(Ser-657)(1:2000),抗PKCα(1:2000 1:2000;Millipore)、抗NR1(1:2000)、抗磷酸化Src家族(Tyr-416)(1:20000)和抗β-微管蛋白(1:5000)。使用增强化学发光检测系统对结合的抗体进行可视化,并使用美国国立卫生研究院图像程序进行半定量分析。 |
| 动物实验 |
Drugs [DM 232 (unifiram) and DM 235 (sunifiram)] were dissolved in isotonic (NaCl 0.9%) saline solution immediately before use. Drug concentrations were prepared so that the necessary dose could be administered in a volume of 10 ml/kg−1 by i.p. injection for mice. For electrophysiological experiments DM 232 was dissolved in dimethylsulfoxide (DMSO) and stock solutions were made to obtain concentrations of DMSO of 0.05% and 0.01% in aCSF, respectively. Control experiments, carried out in parallel for an unrelated project, showed that this concentration of DMSO did not affect the amplitude of synaptic potential. [1]
Electrophysiology [2] Hippocampal slices were prepared as described previously (Moriguchi et al., 2008). Transverse hippocampal slices (400 μm thick) prepared using a vibratome were incubated for 2 h in continuously oxygenized (95% O2, 5% CO2) artificial cerebrospinal fluid (ACSF) at room temperature. After a 2-h recovery period, slices were transferred to an interface recording chamber and perfused at a flow rate of 2 ml/min with ACSF warmed to 34°C. Field excitatory postsynaptic potentials (fEPSPs) were evoked by a 0.05-Hz test stimulus through a bipolar stimulating electrode placed on the Schaffer collateral/commissural pathway and recorded from the stratum radiatum of CA1 region using a glass electrode filled with 3 M NaCl. High-frequency stimulation (HFS) of 100 Hz with a 1-s duration was applied twice with a 10-s interval and test stimulation was continued for the indicated periods. After recording, slices were transferred to a plastic plate cooled on ice to dissect out the CA1 areas. CA1 regions were frozen in liquid nitrogen and stored at –80°C until biochemical analysis was performed. Operation [3] OBX mice were prepared as describe previously [30]. Mice were treated once a day for 7–12 days with sunifiram or sunifiram plus gavestinel starting at 10 days after OBX operation. Behavioral tests were performed at 7–8 days treatment with sunifiram (p.o.) or sunifiram plus gavestinel (i.p.) and electrophysiological and biochemical experiments were performed 9–12 days after sunifiram or sunifiram plus gavestinel treatments. OBX-operated mice had no stereotype killing behavior at least until 3 weeks after operation. However, aggressive behavior without killing behavior was observed in several mice. In the present study, we did not use the aggressive mice separated after OBX surgery for experiment. We breed 3 or 4 mice in each cage and treated the same drug. All animals were sacrificed at the end of experiment and the lesions were verified histologically. Drug treatment [3] Sunifiram was dissolved in (CMC) and administered orally (p.o.) using metal gastric zonde. The dose or volume of injection of sunifiram was 0.1 or 1.0 mg/kg in a volume of 1 ml/100 g body weight. We compared both sunifiram treatment mice and CMC treatment mice (vehicle). Gavestinel was dissolved in tap water and administered intraperitoneal injection (i.p.). The dose or volume of injection of gavestinel was 10 mg/kg. We compared both gavestinel treatment and tap water treatment (vehicle). |
| 参考文献 |
|
| 其他信息 |
Sunifiram is a novel pyrrolidone nootropic drug structurally related to piracetam, which was developed for neurodegenerative disorder like Alzheimer's disease. Sunifiram is known to enhance cognitive function in some behavioral experiments such as Morris water maze task. To address question whether sunifiram affects N-methyl-D-aspartate receptor (NMDAR)-dependent synaptic function in the hippocampal CA1 region, we assessed the effects of sunifiram on NMDAR-dependent long-term potentiation (LTP) by electrophysiology and on phosphorylation of synaptic proteins by immunoblotting analysis. In mouse hippocampal slices, sunifiram at 10-100 nM significantly enhanced LTP in a bell-shaped dose-response relationship which peaked at 10 nM. The enhancement of LTP by sunifiram treatment was inhibited by 7-chloro-kynurenic acid (7-ClKN), an antagonist for glycine-binding site of NMDAR, but not by ifenprodil, an inhibitor for polyamine site of NMDAR. The enhancement of LTP by sunifilam was associated with an increase in phosphorylation of α-amino-3-hydroxy-5-methylisozazole-4-propionate receptor (AMPAR) through activation of calcium/calmodulin-dependent protein kinase II (CaMKII) and an increase in phosphorylation of NMDAR through activation of protein kinase Cα (PKCα). Sunifiram treatments at 1-1000 nM increased the slope of field excitatory postsynaptic potentials (fEPSPs) in a dose-dependent manner. The enhancement was associated with an increase in phosphorylation of AMPAR receptor through activation of CaMKII. Interestingly, under the basal condition, sunifiram treatments increased PKCα (Ser-657) and Src family (Tyr-416) activities with the same bell-shaped dose-response curve as that of LTP peaking at 10 nM. The increase in phosphorylation of PKCα (Ser-657) and Src (Tyr-416) induced by sunifiram was inhibited by 7-ClKN treatment. The LTP enhancement by sunifiram was significantly inhibited by PP2, a Src family inhibitor. Finally, when pretreated with a high concentration of glycine (300 μM), sunifiram treatments failed to potentiate LTP in the CA1 region. Taken together, sunifiram stimulates the glycine-binding site of NMDAR with concomitant PKCα activation through Src kinase. Enhancement of PKCα activity triggers to potentiate hippocampal LTP through CaMKII activation. [2]
This is a first report showing that nootropic drug, sunifiram-enhanced hippocampal LTP via stimulation of the glycine-binding site of NMDAR. The glycine-binding site is present in the NR1 subunit (Wafford et al., 1995) and it is an attractive target site for AD drugs. For example, the ligands for glycine-binding site of NMDAR such as milacemide, a glycine prodrug, and D-cycloserine, a partial glycine agonist, have been clinically tested for use in AD patients (Schwartz et al., 1991, 1996; Dysken et al., 1992). In addition, a partial glycine agonist, D-cycloserine, and a glycine prodrug, milacemide, are known to improve memory deficits in aged rat (Hanndelmann et al., 1989; Baxter et al., 1994). We have previously reported that the other nootropic drug, nefiracetam, enhances synaptic transmission through the glycine-binding site of NMDAR in rat cortical neurons (Moriguchi et al., 2003). We hypothesized that activation of the glycine-binding site of NMDAR improves cognitive function and memory deficits seen in AD patients. In this study, we have demonstrated that the precise molecular mechanism underlying LTP enhancement by sunifiram via the glycine-binding site of NMDAR. The sunifiram-enhanced LTP showed a bell-shaped dose-response relationship that peaked at 10 nM. Bell-shaped increases in PKCα (Ser-657) autophosphorylation and NR1 (Ser-896) phosphorylation were closely associated with the bell-shaped enhancement of LTP by sunifiram in the CA1. Increase in NR1 phosphorylation by PKC are critically important for the LTP enhancement, because NR1 phosphorylation accounts for upregulation of NMDAR function (Tingley et al., 1993). In addition, the enhanced Tyr-416 phosphorylation of Src kinase and the concomitant increased NR2B (Tyr-1472) phosphorylation were also associated with the bell-shaped enhancement of LTP by sunifiram in the CA1. Site of NR2B (Try-1472) is phosphorylated by Src family tyrosine kinases such as Fyn (Nakazawa et al., 2001). Src kinase is activated by NMDAR stimulation via PKC-dependent tyrosine kinases (Kelso et al., 1992; Luttrell et al., 1996; Della Rocca et al., 1997) and is sufficient for LTP induction (Lu et al., 1998). Interestingly, when treated with the Src family inhibitor PP2 in hippocampal CA1 slices, autophosphorylation of PKCα (Ser-657) and phosphorylation of Src family (Tyr-416) failed to be potentiated by sunifiram. In addition, PP2 treatment also inhibited the enhancement of LTP by sunifiram in the CA1. Our data suggested that activation of PKC and Src should be a downstream event following stimulation of the glycine site of NMDARs. We found that sunifiram potentiates fEPSPs in the CA1 region through activation of AMPAR in a dose-dependent manner (1–1,000 nM). Galeotti et al. (2003) reported that sunifiram improves cognitive function via AMPAR activation. In fact, other nootropic drugs also directly activate AMPAR function (Ito et al., 1990; Galeotti et al., 2003; Moriguchi et al., 2003). L-type Ca2+ channels and AMPAR in a high concentration of nootropic compounds are likely mediated by CaMKII activity (Yoshii et al., 1997). Indeed, high concentration of nefiracetam enhanced AMPAR functions through increased CaMKII activity in the cortex (Moriguchi et al., 2003). In this study, sunifiram-enhanced AMPAR function likely through increased CaMKII activity. We also found that PKC-dependent phosphorylation of NR1 contributed to the enhancement of NMDAR function potentiated by sunifiram in the CA1. The bell-shaped increases in PKCα (Ser-657) autophosphorylation and phosphorylation of NR1 (Ser-896) were closely associated with the bell-shaped enhancement of LTP by sunifiram. The activation of PKCα (Ser-657) by sunifiram triggers enhancement of NMDAR functions, because PKCα (Ser-657) phosphorylates the Ser-896 of NMDAR NR1 subunit (Tingley et al., 1997). However, the mechanisms underlying the bell-shaped dose-response curve in activation of NMDAR function and in LTP enhancement by sunifiram are unclear at present. AD is associated with downregulation of brain cholinergic and glutamatergic activities (Greenamyre et al., 1987; Giacobini, 2000). Recently, clinical approaches tried to enhance the cholinergic system using anti-cholinesterases such as donepezil, galantamine, and rivastigmine and neuroprotective action using anti-NMDAR such as memantine. In this study, sunifiram potently enhances NMDAR-dependent LTP via the glycine-binding site of NMDAR in the hippocampus. In addition, nootropics including sunifiram increase acetylcholine release in rat brain (Manetti et al., 2000). Our hypothesis is that enhancement of both cholinergic and glutamatergic activities by sunifiram seems to improve cognitive deficits in AD patients. In conclusion, the present study demonstrated that sunifiram treatment markedly enhances hippocampal LTP via glycine-binding site of NMDAR. Sunifiram stimulates the glycine-binding site of NMDAR through PKCα and Src kinase activation. Enhancement of PKCα activity triggers to potentiate hippocampal LTP through CaMKII-mediated NMDAR activation. Enhancement of hippocampal LTP by sunifiram forms the basis of the improvement of cognitive and memory deficits seen in AD patients. [2] |
| 分子式 |
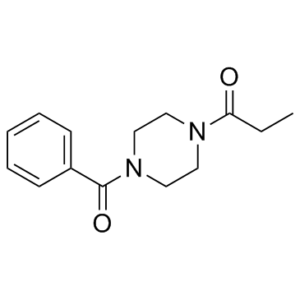
C14H18N2O2
|
|
|---|---|---|
| 分子量 |
246.31
|
|
| 精确质量 |
246.136
|
|
| 元素分析 |
C, 68.27; H, 7.37; N, 11.37; O, 12.99
|
|
| CAS号 |
314728-85-3
|
|
| 相关CAS号 |
|
|
| PubChem CID |
4223812
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
442.0±38.0 °C at 760 mmHg
|
|
| 闪点 |
205.0±19.1 °C
|
|
| 蒸汽压 |
0.0±1.1 mmHg at 25°C
|
|
| 折射率 |
1.561
|
|
| LogP |
0.26
|
|
| tPSA |
40.62
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
2
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
18
|
|
| 分子复杂度/Complexity |
303
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
DGOWDUFJCINDGI-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C14H18N2O2/c1-2-13(17)15-8-10-16(11-9-15)14(18)12-6-4-3-5-7-12/h3-7H,2,8-11H2,1H3
|
|
| 化学名 |
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 50 mg/mL (203.00 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.0599 mL | 20.2996 mL | 40.5992 mL | |
| 5 mM | 0.8120 mL | 4.0599 mL | 8.1198 mL | |
| 10 mM | 0.4060 mL | 2.0300 mL | 4.0599 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。