| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
H2S-releasing compound
|
|---|---|
| 体外研究 (In Vitro) |
S-炔丙基半胱氨酸(SPRC),也称为ZYZ-802,是S-烯丙基半胱氨酸(SAC)的结构类似物,SAC是陈年大蒜提取物中含量最丰富的成分。通过将SAC中的烯丙基改为SPRC中的炔丙基,SPRC成为含有硫原子的氨基酸半胱氨酸的衍生物。SPRC和SAC的另一个类似物是S-丙基半胱氨酸(SPC),其半胱氨酸结构中有丙基。SPRC的药物配方已在乳糖、微晶纤维素和交联聚维酮等填充剂的混合物中进行了研究,显示出良好的流动性和扩大生产的可能性。发明了SPRC(CR-SPRC)和益母草SPRC的控释制剂,并分别在心力衰竭和缺氧损伤中显示出良好的药理作用。SPRC的药理作用机制已被揭示,SPRC减少了Ca2+的积累,激活了抗氧化剂,抑制了STAT3,减少了炎性细胞因子,提高了p53和Bax。在动脉粥样硬化、高血压和其他疾病中,将发现更多的SPRC药理作用和机制。[2]
|
| 体内研究 (In Vivo) |
硫化氢(H(2)S)是一种新型的气态信使,由L-半胱氨酸通过两种吡哆醛-5'-磷酸依赖酶胱硫醚β-合酶(CBS)和胱硫醚γ-裂解酶(CSE)内源性合成。S-炔丙基半胱氨酸(SPRC)是一种缓慢释放H(2)S的药物,提供半胱氨酸,半胱氨酸是CSE的底物。本研究旨在探讨SPRC在小鼠急性胰腺炎(AP)体内模型中的作用。通过每小时注射50µg/kg的蓝蛙素10小时在小鼠体内诱导AP。用SPRC(10mg/kg)或赋形剂(蒸馏水)治疗小鼠。在胰腺炎诱导前12小时或3小时给予SPRC。在最后一次注射蓝蛙素后1小时处死小鼠。采集血液、胰腺和肺组织并进行处理,以测量胰腺和肺中的血浆淀粉酶、血浆H(2)S、髓过氧化物酶(MPO)活性和细胞因子水平。结果显示,胰腺和肺部炎症的显著减少与AP诱导前3小时给予SPRC有关。此外,SPRC的有益作用与胰腺和肺促炎细胞因子的减少以及抗炎细胞因子的增加有关。在AP诱导前12小时给予SPRC并没有显著改善胰腺和肺部炎症。对照组、赋形剂组和SPRC(AP前3小时给药)治疗组的血浆H(2)S浓度存在显著差异。综上所述,这些数据为SPRC在AP中的保护作用提供了证据,这可能是由于其缓慢释放内源性H(2)S。[1]
|
| 酶活实验 |
使用DuoSet ELISA试剂盒通过夹心ELISA测定胰腺和肺组织匀浆中细胞因子(IL-1β、IL-6、IL-10和TNF-α)的水平。简而言之,将抗细胞因子一抗涂覆在96孔ELISA板上,并在室温下孵育过夜。将样品和标准品加入孔中并孵育2小时,洗涤孔,加入生物素化的山羊抗小鼠细胞因子抗体2小时。再次洗涤板,加入与HRP结合的链霉抗生物素蛋白抗体20分钟。进一步洗涤后,加入TMB进行显色,用2 N H2SO4终止反应。在450nm处测量吸光度。根据标准曲线估算样品的细胞因子浓度。然后根据组织的DNA含量校正细胞因子浓度[2]。
|
| 动物实验 |
Mice were randomly assigned to four groups (n = 10 per group). Group 1: Animals were given hourly intraperitoneal (i.p.) injections of normal saline (CTRL group). Group 2: Animals were treated with distilled water (DW) followed by hourly i.p. injections of caerulein (50 µg/kg) over 10 h to induce AP (Veh+Cae). Group 3: Animals were treated with SPRC (10 mg/kg), 3 h before hourly injections of caerulein (50 µg/kg) over 10 h (SPRC 3 h+Cae). Group 4: Animals were treated with SPRC (10 mg/kg), 12 h before hourly injections of caerulein (50 µg/kg) over 10 h (SPRC 12 h+Cae). SPRC was dissolved in DW. One hour after the last caerulein injection animals were sacrificed by an i.p. injection of a lethal dose of pentobarbital (50 mg/kg: Nembutal). Blood, pancreas and lung tissues were collected. Samples of pancreas and lung were weighed, snap frozen in liquid nitrogen and then stored at −80°C for subsequent measurement of tissue myeloperoxidase (MPO) activities, and cytokines as described in detail below. Harvested heparinized blood was centrifuged (10,000 rpm, 10 min, 4°C) and the plasma was aspirated and stored at −80°C for subsequent detection of plasma amylase and H2S. Parts of the pancreas and lung were also fixed in 10% v/v neutral phosphate-buffered formalin for more than 48 h and then were processed for histology.[2]
|
| 参考文献 | |
| 其他信息 |
Furthermore, we investigated if SPRC treatment affected plasma H2S levels in AP and the mechanism by which inflammation was modulated by SPRC. SPRC, like SAC, could potentially influence the synthesis of endogenous H2S. In vitro, SPRC was found to increase H2S synthesizing enzyme activity in normal pancreatic acini compared to the vehicle control, but the difference was not significant in the presence of caerulein hyper-stimulation (unpublished data). However, while we observed a significant increase in plasma H2S concentration in AP-induced mice treated with vehicle, SPRC injected 3 h before AP lowered this increase. As expected, high plasma H2S concentration observed in vehicle treated mice after induction of AP was pro-inflammatory and damaging. Administration of SPRC 3 h before AP induction and thus the slow release of H2S could have inhibited CSE by a feedback mechanism resulting in significantly lower levels of H2S compared to the vehicle treated mice. Administration of a slow H2S releasing compound has previously been shown to be associated with a decrease in endogenous H2S formation. Evidently, further studies are needed to explore the mechanisms of SPRC effects in AP in detail.
In conclusion, SPRC 10 mg/kg injected 3 h prior to induction of AP ameliorated the disease by reducing the inflammatory cell infiltration in pancreas and lung and by modulating pro- and anti-inflammatory cytokine profile in plasma. Thus SPRC provides a valuable lead for the treatment of AP. It could be postulated that the beneficial effects of SPRC in AP could be by virtue of its slow release of endogenous H2S and a possible negative feedback mechanism on CSE.[2] |
| 分子式 |
C6H9NO2S
|
|---|---|
| 分子量 |
159.2
|
| 精确质量 |
159.035
|
| 元素分析 |
C, 45.27; H, 5.70; N, 8.80; O, 20.10; S, 20.14
|
| CAS号 |
3262-64-4
|
| PubChem CID |
22789047
|
| 外观&性状 |
Typically exists as Off-white to yellow solids at room temperature
|
| 密度 |
1.284g/cm3
|
| 沸点 |
294.8ºC at 760 mmHg
|
| 熔点 |
176-178 °C
|
| 闪点 |
132.1ºC
|
| 折射率 |
1.57
|
| LogP |
0.465
|
| tPSA |
88.62
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
10
|
| 分子复杂度/Complexity |
160
|
| 定义原子立体中心数目 |
1
|
| SMILES |
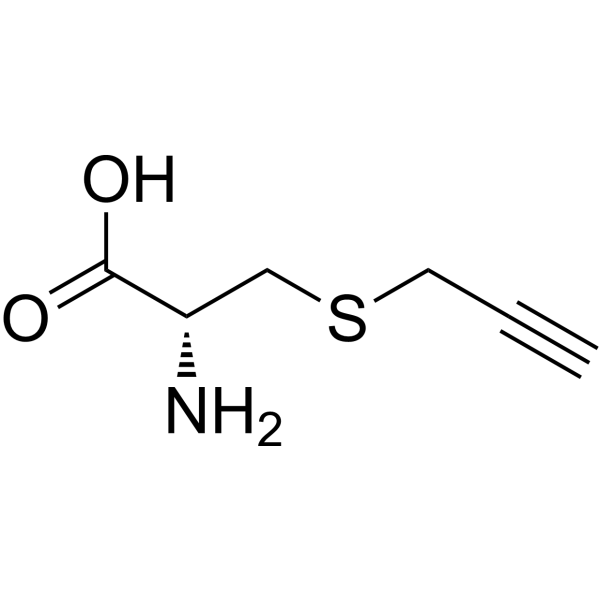
C#CCSC[C@@H](C(=O)O)N
|
| InChi Key |
JAKVEOCMEMGHGB-YFKPBYRVSA-N
|
| InChi Code |
InChI=1S/C6H9NO2S/c1-2-3-10-4-5(7)6(8)9/h1,5H,3-4,7H2,(H,8,9)/t5-/m0/s1
|
| 化学名 |
(2R)-2-amino-3-prop-2-ynylsulfanylpropanoic acid
|
| 别名 |
S-Propargyl-cysteine; (R)-2-Amino-3-(2-propynylthio)propanoic Acid; (R)-2-Amino-3-(prop-2-yn-1-ylthio)propanoic acid; s-propargyl-cysteine; (R)-2-Amino-3-(prop-2-ynylthio)propanoic acid; SPRC; s-propargylcysteine; (L)-3-(PROPARGYLSULFENYL)-ALANINE; SPRC
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ~25 mg/mL (~157.03 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 6.2814 mL | 31.4070 mL | 62.8141 mL | |
| 5 mM | 1.2563 mL | 6.2814 mL | 12.5628 mL | |
| 10 mM | 0.6281 mL | 3.1407 mL | 6.2814 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。