| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
Macrolide;Toxoplasma
|
|---|---|
| 体外研究 (In Vitro) |
用螺旋霉素(24小时;1-1000 μM;弓形虫感染的HeLa细胞和HeLa细胞)处理可降低细胞毒性并表现出抗弓形虫活性,HeLa细胞的IC50值为189 μM,弓形虫的IC50值为262 μM -感染的HeLa细胞[3]。
|
| 体内研究 (In Vivo) |
用螺旋霉素(100 mg/kg;腹腔注射;每天;持续 4 天;雌性 KM 小鼠)治疗可减少速殖子和肝毒性,并大大增强抗氧化作用。吡霉素治疗还可以减轻肝脏的肉芽肿性炎症[3]。
|
| 细胞实验 |
细胞系:弓形虫感染的HeLa细胞和HeLa细胞
浓度:1-1000 μM 孵育时间:24小时 结果:细胞毒性降低。 |
| 动物实验 |
Animal Model: 36 female KM mice with T.gondii[3]
Dosage: 100 mg/kg Administration: Intraperitoneal injection; every day; for 4 days Result: Tachyzoites were considerably fewer in number. decreased hepatotoxicity and markedly increased antioxidative benefits. Formation of cysts and granulomas was inhibited. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The extent of absorption of Spiramycin was shown to be incomplete. Oral bioavailability ranges from 30-39%. Spiramycin has slower rate of absorption than Erythromycin. It has a high pKa (7.9) which could be a result of high degree of ionization in acidic medium of the stomach. Fecal-biliary route is the primary route of elimination. The secondary route is renal-urinary route. The tissue distribution of spiramycin is extensive. The volume of distribution is in excess of 300 L, and concentrations achieved in bone, muscle, respiratory tract and saliva exceed those found in serum. Spiramycin showed high concentrations in tissues such as: lungs, bronchi, tonsils, and sinuses. 80% of the administered dose excreted in the bile, which makes the fecal-biliary route is the most important route of elimination. Enterohepatic recycling could also occur. Only 4 to 14% of an administered dose is eliminated through renal-urinary excretion route. Spiramycin is well absorbed in humans after oral administration. Oral administration of 15-30 mg/kg bw to healthy young male adults resulted in peak plasma levels in 3-4 hours and plasma concentrations of 0.96-1.65 mg/l. After intravenous dosing (7.25 mg/kg b.w.) a large volume of distribution (Vdss 5.6 l/kg) was observed indicating extensive tissue distribution. Biotransformation did not appear to be important. Biliary excretion was the main route of excretion; only 7-20% of an oral dose was excreted in the urine. Spiramycin is known to achieve high tissue:serum concentrations in pulmonary and prostatic tissues, and in skin. Spiramycin crosses the placenta to the fetus. Concns of the antibiotic in maternal serum, cord blood, & the placenta after a dosage regimen of 2 g/day were 1.19 ug/ml, 0.63 ug/ml, & 2.75 ug/ml, respectively. When the maternal dose was increased to 3 g/day, the levels were 1.69 ug/ml, 0.78 ug/ml, & 6.2 ug/ml, respectively. Based on these results, the cord:maternal serum ratio is approx 0.5. Moreover, at these doses, spiramycin is concentrated in the placenta with levels approx 2-4 times those in the maternal serum. ... Spiramycin is excreted into breast milk. Nursing infants of mothers receiving 1.5 g/day for 3 days had spiramycin serum concns of 20 ug/ml. This concn was bacteriostatic. /MILK/ Spiramycin is a macrolide antibiotic that is active against most of the microorganisms isolated from the milk of mastitic cows. This work investigated the disposition of spiramycin in plasma & milk after iv, intramuscular & subcutaneous admin. Twelve healthy cows were given a single injection of spiramycin at a dose of 30,000 IU/kg by each route. Plasma & milk were collected post injection. Spiramycin concn in the plasma was determined by a high performance liquid chromatography method, & in the milk by a microbiological method. The mean residence time after iv admin was significantly longer (P<0.01) in the milk (20.7 +/- 2.7 h) than in plasma (4.0 +/- 1.6 h). An average milk-to-plasma ratio of 36.5 +/- 15 was calculated from the area concn-time curves. Several pharmacokinetic parameters were examined to determine the bioequivalence of the two extravascular routes. The dose fraction adsorbed after intramuscular or subcutaneous admin was almost 100% & was bioequivalent for the extravascular routes, but the rates of absorption, the max concns & the time to obtain them differed significantly between the two routes. Spiramycin quantities excreted in milk did not differ between the two extravascular routes but the latter were not bioequivalent for max concn in the milk. However, the two routes were bio-equivalent for the duration of time the milk concn exceeded the minimal inhibitory concn (MIC) of various pathogens causing infections in the mammary gland. Plasma protein binding ranges from 10 to 25%. An oral dose of 6 million units produces peak blood concentrations of 3.3 ug/mL after 1.5 to 3 hours; the half life is about 5 to 8 hours. High tissue concentrations are achieved and persist long after the plasma concentration has fallen to low levels. For more Absorption, Distribution and Excretion (Complete) data for SPIRAMYCIN (13 total), please visit the HSDB record page. Metabolism / Metabolites Spiramycin is less metabolised than some of the other macrolides. Metabolism has not been well studied. It is mainly done in the liver to the active metabolites. In cattle, the metabolite neospiramycin, the demycarosyl derivative, is formed. Concentrations of neospiramycin in muscle and kidney were marginally higher than those of spiramycin 14-28 days after dosing; in muscle, levels of neospiramycin and spiramycin were approximately equal. Spiramycin is metabolized in the liver to active metabolites; substantial amounts are excreted in the bile and about 10% in the urine. Biological Half-Life Intravenous: Young persons (18 to 32 years of age): Approximately 4.5 to 6.2 hours. Elderly persons (73 to 85 years of age): Approximately 9.8 to 13.5 hours. Oral: 5.5-8 hours, Rectal in children: 8 hours An oral dose of 6 million units produces ... /a/ half life is about 5 to 8 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Spiramycin is a macrolide antibiotic used for the treatment and control of a number of bacterial and mycoplasmal infections in animals. It is available as a spiramycin embonate for use in animal feed, and as the adipate, a more soluble form, for administration by other routes. It has also been used in the protozoal infections cryptosporidiosis and toxoplasmosis. HUMAN EXPOSURE AND TOXICITY: Spiramycin is reported to cause contact dermatitis in occupational settings. A man who worked in a feed factory developed allergic contact dermatitis due to airborne spiramycin. The patient suffered recurrent outbreaks of eczematous lesions on uncovered areas during working periods. Spiramycin is also reported to cause hypersensitivity reactions. Rhinoconjunctivitis and spasmodic cough are reported in a 34 year-old female handling spiramycin powder in a pharmaceutical factory. The symptoms appeared within the first few hours of coming into contact with the drug and continued for several hours after leaving her place of work. One year after starting work in the pharmaceutical industry a 35-year-old non-atopic maintenance engineer developed attacks of sneezing, coughing and breathlessness. Inhalation challenge tests carried out in the hospital with gradually increasing quantities of spiramycin reproduced his symptoms and led to the development of late asthmatic reactions. Additionally, two cases of bronchial asthma due to spiramycin in workers of a pharmaceutical factory were reported. The subjects complained of cough, breathlessness and symptoms of asthma at work when coming into contact with spiramycin's powder. The symptoms cleared when away from work for more than 3 or 4 days. ANIMAL STUDIES: Groups of 2 male and 2 female monkeys (Macaca fascicularis) were given daily intravenous injections of 0, 240,000, 360,000, and 540,000 iu/kg bw/day spiramycin adipate for 5 days. Hypersalivation occurred during injection in all dose groups. Muscle hypotonia and nauseous spasticity occurred in several high dose monkeys and in one given the low dose. No abnormalities of body weights occurred but food consumption was reduced in all treated animals. A slight decrease in hemoglobin, red cell numbers and hematocrit was noted in high dose animals. In a short-term dietary study in which rats were given the equivalent of up to 3900 mg/kg bw for 13 weeks, the only major effects noted were a reduction in neutrophil counts in some mid- and high-dose animals, and the dilatation of the caecum. In another dietary study in the rat, animals were given up to the equivalent of 720 mg/kg bw/day for one year. The only notable effects were reductions in the body weights of females receiving the high doses, and increases in relative liver, kidney, and adrenal weights at high dose levels in animals of both sexes. Hepatic glycogen depletion occurred at all dose levels but not in controls. In mongrel dogs given 500 mg/kg bw/day for up to 56 days, reductions in spermatogenesis and testicular atrophy occurred. Kidney damage was also seen. When beagles were given orally spiramycin at up to the equivalent of 150 mg/kg bw/day for two years, testicular damage was not seen although degenerative changes occurred in other organs. In teratogenicity studies in mice, oral doses of spiramycin of up to 400 mg/kg bw given over days 5-15 of gestation had no effects on the outcome of pregnancy. intravenous doses of up to 84 mg/kg bw/day given on days 6-15 of gestation to rats and day 6-19 to rabbits had no effect on developmental, but oral dose of 200 and 400 mg/kg bw/day in rabbit produced caecal enlargement in mothers. Groups of 20 pregnant rats were treated intravenously on days 6-15 of gestation with doses of 0, 90 000, 180 000, and 270 000 iu/kg bw/day with spiramycin adipate. The highest dose given produced brief (5 minutes) ataxia and tremors immediately after dosing. A slight but significant reduction in fetal weight occurred at the intermediate dose but all values were within historical control ranges. There were no increased incidences of any fetal anomaly noted in this study. In a study where male rats were given doses of 30 mg/kg bw/day for 8 days by an unspecified route, mitotic and meiotic abnormalities in spermatogonia were noted. Negative result were obtained with spiramycin adipate and embonate in a forward-mutation test in mammalian cells in vitro, in an in vitro cytogenic assay, and in the mouse micronucleus test. Protein Binding Low level of protein binding (10-25%). Interactions The macrolide antibiotics include natural members, prodrugs & semisynthetic derivatives. These drugs are indicated in a variety of infections & are often combined with other drug therapies, thus creating the potential for pharmacokinetic interactions. Macrolides can both inhibit drug metab in the liver by complex formation & inactivation of microsomal drug oxidising enzymes & also interfere with microorganisms of the enteric flora through their antibiotic effects. Over the past 20 yrs, a number of reports have incriminated macrolides as a potential source of clinically severe drug interactions. However, differences have been found between the various macrolides in this regard & not all macrolides are responsible for drug interactions. With the recent advent of many semisynthetic macrolide antibiotics it is now evident that they may be classified into 3 different groups in causing drug interactions. The first group (e.g. troleandomycin, erythromycins) are those prone to forming nitrosoalkanes & the consequent formation of inactive cytochrome P450-metabolite complexes. The second group (e.g. josamycin, flurithromycin, roxithromycin, clarithromycin, miocamycin & midecamycin) form complexes to a lesser extent & rarely produce drug interactions. The last group (e.g. spiramycin, rokitamycin, dirithromycin & azithromycin) do not inactivate cytochrome P450 & are unable to modify the pharmacokinetics of other cmpds. It appears that 2 structural factors are important for a macrolide antibiotic to lead to the induction of cytochrome P450 & the formation in vivo or in vitro of an inhibitory cytochrome P450-iron-nitrosoalkane metabolite complex: the presence in the macrolide molecules of a non-hindered readily accessible N-dimethylamino group & the hydrophobic character of the drug. Troleandomycin ranks first as a potent inhibitor of microsomal liver enzymes, causing a significant decr of the metab of methylprednisolone, theophylline, carbamazepine, phenazone (antipyrine) & triazolam. Troleandomycin can cause ergotism in patients receiving ergot alkaloids & cholestatic jaundice in those taking oral contraceptives. Erythromycin & its different prodrugs appear to be less potent inhibitors of drug metab. Case reports & controlled studies have, however, shown that erythromycins may interact with theophylline, carbamazepine, methylprednisolone, warfarin, cyclosporin, triazolam, midazolam, alfentanil, disopyramide & bromocriptine, decreasing drug clearance. The bioavailability of digoxin appears also to be increased by erythromycin in patients excreting high amounts of reduced digoxin metabolites, probably due to destruction of enteric flora responsible for the formation of these cmpds. These incriminated macrolide antibiotics should not be administered concomitantly with other drugs known to be affected metabolically by them, or at the very least, combined admin should be carried out only with careful patient monitoring. Reduced plasma concentrations of levodopa have been reported when given with spiramycin. The authors report the case of a 21 year old woman with a congenital long QT syndrome who had several syncopal attacks at least one of which was caused by torsades de pointes. This sudden complication was attributed to the simultaneous prescription of Spiramycine and Mequitazine over a 48 hour period. These two drugs are not considered to be predisposing factors for torsades de pointes despite the fact that they belong to two families of drugs which can trigger this type of arrhythmia. The withdrawal of this treatment led to the complete regression of the syncopal episodes with a follow-up of two years and a significant shortening of the initial QTc interval which remained, nevertheless, longer than normal. This case underlines the potential risks of drug associations of these two families of drugs, especially in patients with the congenital long QT syndrome. Non-Human Toxicity Values LD50 Rat oral 3550 mg/kg LD50 Rat ip 575 mg/kg LD50 Rat sc 1 g/kg LD50 Rat iv 170 mg/kg For more Non-Human Toxicity Values (Complete) data for SPIRAMYCIN (23 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Spiramycin is a primarily bacteriostatic macrolide antimicrobial agent with activity against Gram-positive cocci and rods, Gram-negative cocci and also Legionellae, mycoplasmas, chlamydiae, some types of spirochetes, Toxoplasma gondii and Cryptosporidium. Spiramycin is a 16-membered ring macrolide discovered in 1952 as a product of Streptomyces ambofaciens that has been available in oral formulations since 1955, and parenteral formulations since 1987. Resistant organisms include Enterobacteria, pseudomonads, and moulds.
Spiramycin is a macrolide originally discovered as product of Streptomyces ambofaciens, with antibacterial and antiparasitic activities. Although the specific mechanism of action has not been characterized, spiramycin likely inhibits protein synthesis by binding to the 50S subunit of the bacterial ribosome. This agent also prevents placental transmission of toxoplasmosis presumably through a different mechanism, which has not yet been characterized. Drug Indication Macrolide antibiotic for treatment of various infections. Mechanism of Action The mechanism of action of macrolides has been a matter of controversy for some time. Spiramycin, a 16-membered macrolide, inhibits translocation by binding to bacterial 50S ribosomal subunits with an apparent 1 : 1 stoichiometry. This antibiotic is a potent inhibitor of the binding to the ribosome of both donor and acceptor substrates. The primary mechanism of action is done by stimulation of dissociation of peptidyl-tRNA from ribosomes during translocation.I Therapeutic Uses Anti-Bacterial Agents; Coccidiostats /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Spiramycin is included in the database. MEDICATION (VET): Spiramycin is a macrolide antibiotic used for the treatment and control of a number of bacterial and mycoplasmal infections in animals. It is available as a spiramycin embonate for use in animal feed, and as the adipate, a more soluble form, for administration by other routes. The aim of this study is to evaluate the efficacy of spiramycin in prevention of mother-to-child transmission of Toxoplasma gondii infection. Patients within first trimester of their pregnancy with Toxoplasma IgM positivity (>0.65 index, ELISA, VIDAS) and IgG positivity (>8 IU/ml), who had low IgG avidity (<0.50 index, ELISA, Architet) were considered as having acute toxoplasmosis. These patients who had amniocentesis at the 19th-21st week of pregnancy were examined for the detection of Toxoplasma DNA. Detailed ultrasonographic examinations performed between the 20th and 24th gestational weeks and the mothers and babies were followed for at least one year. ut of 61 patients, 55 (90.2%) had received Spy prophylaxis while 6 (9.8%) cases refused Spy prophylaxis. Toxoplasma PCR test was found to be positive in amniotic fluid of 4 (6.6%) patients obtained by amniocentesis at the 19th-21st week of pregnancy. All four of these patients had refused Spy prophylaxis had positive Toxoplasma PCR in amniotic fluid (p < 0.01). Our results seem to encourage the use of spiramycin in women with toxoplasmosis during pregnancy. Spiramycin is a macrolide antibacterial that is used similarly to erythromycin in the treatment of susceptible bacterial infections. It has also been used in the protozoal infections cryptosporidiosis and toxoplasmosis. Drug Warnings The most frequent adverse effects are gastrointestinal disturbances. Transient parethesia has been reported during parenteral use. Spiramycin, a 16-membered lactone ring macrolide, has been in clinical use for the past 15 years with little serious associated toxicity. GI disturbance has usually been mild & no changes in GI motility have been noted either experimentally or in humans, in contrast to other macrolides, such as erythromycin. Allergic reactions have been uncommon & mainly restricted to transient skin eruptions. Although liver injury is a possible complication of most macrolide treatments, no conclusive evidence for spiramycin-induced hepatitis is currently available, and, again in contrast to most other macrolides, the lack of drug interactions with spiramycin has been clearly established in biochemical, pharmacokinetic & clinical studies. ... Allergic drug reactions to macrolides are extremely rare & there is little information in the literature concerning relevant diagnostic tests. ... Twenty-one patients were recently seen for assumed allergies (principally urticaria) to diverse macrolides. Skin tests (prick & intradermal tests) were performed with injectable forms of spiramycin & erythromycin. Seventeen out of 21 patients were provoked under strict hospital surveillance. ... Only 3 patients had a positive provocation test & were thus truly allergic (to spiromycin). They had positive skin tests to both macrolides tested. ... Most hypersensitivity reactions to macrolides are therefore diagnosed with provocation tests. We recently reported two cases of QT interval prolongation & cardiac arrest in newborns receiving antibiotic therapy with spiramycin, a macrolide agent extensively used for toxoplasmosis prophylaxis. In this study we assessed the effects of this drug on ventricular repolarization & on the potential risk of lethal arrhythmias in 8 newborn infants in whom toxoplasmosis prophylaxis after birth was necessary. Electrocardiograms (ECGs) & echocardiograms were recorded during spiramycin therapy (350,000 i.u./kg/ day) & after its withdrawal. In a control group of 8 healthy newborns matched for age & sex, no differences were found between 2 ECGs analogously recorded. The QT interval corrected for heart rate (QTc) was longer during spiramycin therapy than after drug withdrawal (448 +/- 32 msec vs 412 +/- 10 msec, +9%, p=0.021). QTc dispersion, expressed as the difference between the longest & the shortest value in 12 different leads (QTcmax-min), was also higher during spiramycin therapy (60 +/- 32 msec vs 34 +/- 8 msec, +76%, p=0.021), mainly because of a major lengthening of the longest QTc (QTcmax). QTc & QTc dispersion were markedly increased in the 2 newborns who experienced cardiac arrest after beginning treatment compared with the 6 neonates who had no drug-induced symptoms. During therapy 7 of 8 newborns had a rare abnormality in the thickening of the left ventricular posterior wall similar to that observed in patients with congenital long QT syndrome. This abnormality disappeared after drug withdrawal. Thus antibiotic therapy with spiramycin in the neonatal period may induce QT interval prolongation & incr QT dispersion. When this effect on ventricular repolarization is more marked, it may favor the occurrence of torsades des pointes & lead to cardiac arrest. Pharmacodynamics The absolute bioavailability of oral spiramycin is generally within the range of 30 to 40%. After a 1 g oral dose, the maximum serum drug concentration was found to be within the range 0.4 to 1.4 mg/L. |
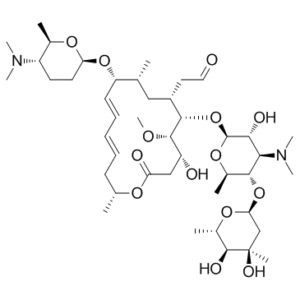
| 分子式 |
C43H74N2O14
|
|---|---|
| 分子量 |
843.0527
|
| 精确质量 |
842.513
|
| 元素分析 |
C, 61.26; H, 8.85; N, 3.32; O, 26.57
|
| CAS号 |
8025-81-8
|
| 相关CAS号 |
8025-81-8;24916-52-7 (III);67724-08-7 (Embonate);68880-55-7 (adipate);
|
| PubChem CID |
5289394
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
913.7±65.0 °C at 760 mmHg
|
| 闪点 |
506.4±34.3 °C
|
| 蒸汽压 |
0.0±0.6 mmHg at 25°C
|
| 折射率 |
1.550
|
| LogP |
3.06
|
| tPSA |
195
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
16
|
| 可旋转键数目(RBC) |
11
|
| 重原子数目 |
59
|
| 分子复杂度/Complexity |
1370
|
| 定义原子立体中心数目 |
19
|
| SMILES |
O1[C@@]([H])(C([H])([H])[H])[C@@]([H])([C@@]([H])([C@@]([H])([C@@]1([H])O[C@]1([H])[C@]([H])([C@@]([H])(C([H])([H])C(=O)O[C@]([H])(C([H])([H])[H])C([H])([H])C([H])=C([H])C([H])=C([H])[C@@]([H])([C@]([H])(C([H])([H])[H])C([H])([H])[C@]1([H])C([H])([H])C([H])=O)O[C@@]1([H])C([H])([H])C([H])([H])[C@@]([H])([C@]([H])(C([H])([H])[H])O1)N(C([H])([H])[H])C([H])([H])[H])O[H])OC([H])([H])[H])O[H])N(C([H])([H])[H])C([H])([H])[H])O[C@]1([H])C([H])([H])[C@@](C([H])([H])[H])([C@@]([H])([C@@]([H])(C([H])([H])[H])O1)O[H])O[H] |c:39,43|
|
| InChi Key |
ACTOXUHEUCPTEW-AQKFJFIXSA-N
|
| InChi Code |
InChI=1S/C43H74N2O14/c1-24-21-29(19-20-46)39(59-42-37(49)36(45(9)10)38(27(4)56-42)58-35-23-43(6,51)41(50)28(5)55-35)40(52-11)31(47)22-33(48)53-25(2)15-13-12-14-16-32(24)57-34-18-17-30(44(7)8)26(3)54-34/h12-14,16,20,24-32,34-42,47,49-51H,15,17-19,21-23H2,1-11H3/b13-12+,16-14+/t24-,25-,26-,27-,28+,29+,30+,31-,32+,34+,35+,36-,37-,38-,39+,40+,41+,42+,43?/m1/s1
|
| 化学名 |
2-[(4R,5S,6S,7R,9R,10R,11E,13E,16R)-6-{[(2S,3R,4R,5S,6R)-5-{[(2S,5S,6S)-4,5-dihydroxy-4,6-dimethyloxan-2-yl]oxy}-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy}-10-{[(2R,5S,6R)-5-(dimethylamino)-6-methyloxan-2-yl]oxy}-4-hydroxy-5-methoxy-9,16-dimethyl-2-oxo-1-oxacyclohexadeca-11,13-dien-7-yl]acetaldehyde
|
| 别名 |
HSDB-7027; Rovamycin; HSDB7027; HSDB 7027
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 100~157 mg/mL ( 118.62~186.22 mM )
Ethanol : ~157 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (2.97 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (2.97 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (2.97 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.5 mg/mL (2.97 mM) 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.1862 mL | 5.9308 mL | 11.8617 mL | |
| 5 mM | 0.2372 mL | 1.1862 mL | 2.3723 mL | |
| 10 mM | 0.1186 mL | 0.5931 mL | 1.1862 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|
|