| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10g |
|
||
| 25g |
|
||
| Other Sizes |
|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Sodium lauryl sulfate is a white or cream-colored crystal, flake, or powder with a faint odor. Sodium dodecyl sulfate is used in general as a detergent, dispersant, and surfactant. Pure sodium dodecyl sulfate is used mainly in dentifrice products (a powder, paste, or liquid for cleaning the teeth), in hair shampoos, and in emulsion polymerization. The rest is either used in special cosmetic formulations, e.g. for bubble baths and hair bleaches, or as a fine chemical, e.g. as denaturing agent in gel electrophoresis. Besides pure sodium dodecyl sulfate detergent, manufacturers usually produce "technical grade" sodium dodecyl sulfate, a product that consists of approximately 70 % sodium dodecyl sulfate and 30 % sodium tetradecyl sulfate. This product is generally called sodium lauryl sulfate. Technical grade sodium dodecyl sulfate is used as a detergent in dish-washing products (main use), as additive for plastics and latices, and in paints and lacquers. Sodium lauryl sulfate is used as a flea and tick repellant in one registered pesticide product--a flea and tick shampoo for cats and dogs. Sodium lauryl sulfate also is a widely used component of many nonpesticidal consumer products currently marketed in the United States, including shampoos and fruit juices. It is also used in hydraulic fracturing to prevent the formation of emulsions in the fracture fluid. HUMAN EXPOSURE AND TOXICITY: Among 242 patients suffering from eczematous dermatitis, the percentage of allergic reactions reached 54.6%. A great number of allergic reactions to sodium lauryl sulfate (6.4%) was observed. The study was conducted to compare the effects of sodium lauryl sulfate (SLS)-free and SLS-containing dentifrice (a powder, paste, or liquid for cleaning the teeth) in patients with recurrent aphthous stomatitis (RAS). Although SLS-free products did not reduce the number of ulcers and episodes, it affected the ulcer-healing process and reduces pain in daily lives in patients with RAS. ANIMAL STUDIES: The repeated dose toxicity of sodium dodecyl sulphate was studied extensively. Tests range from sub-acute (28 days) to chronic (2 years in rat) studies. Further, the substance was tested in two different species (rat and dog) and by means of two different routes of administration (diet and gavage). The primary effect of sodium dodecyl sulphate in animals is a local irritation of the gastro-intestinal tract. Developmental toxicity/teratogenicity of sodium dodecyl sulphate was investigated in three different species. Mice and rabbits are the most sensitive species. Sodium dodecyl sulphate was extensively tested for genetic toxicity. Neither the bacterial tests nor the various tests in mammalian systems (in vitro and in vivo) have shown any indication of genotoxicity with or without metabolic activation. Published reports suggest that sodium lauryl sulfate has low acute mammalian toxicity and no known chronic effects. ECOTOXICITY STUDIES: Uptake, tissue distribution, and elimination of lauryl sulfate were investigated in carp. The chemical concentrated in hepatopancreas and gallbladder. Maximum whole-body levels were reached during 24-72 hr. Survival time decreased with increased water hardness. Sodium lauryl sulfate has been tested as a repellent against several species of sharks. It did not provoke a repellency response at a low enough concentration to function effectively as a classical, surrounding-cloud type, repellent. The range of potency, however, does allow it to be used as a directional repellent. Toxicity Data LC50 (rat) > 3,900 mg/m3/1h Interactions Contact allergy to fragrance chemicals is an increasing problem. Polysensitization is likely to be a phenotype of increased susceptibility to contact allergy. The factors that play a role in polysensitization are largely unknown. Identifying these risk factors is important with regard to future studies on the aetiology of contact allergy. To investigate whether enhanced skin irritability is a risk factor for the development of polysensitization to fragrance chemicals, one hundred participants characterized by fragrance contact allergy were included in /this/ study. The participants were patch tested on the back with 25 individual fragrance chemicals and fragrance mixes... and on the upper arm with sodium lauryl sulfate (SLS) (consisting of SLS concentrations of 0.45%, 0.67%, 1%, and 1.5%). Reading of both tests was performed during the following visits at days 2, 3, and 7. The response to SLS was monitored by measuring transepidermal water loss (TEWL). Polysensitization was defined as three or more allergic reactions to non-cross-reacting fragrance chemical allergens. Individuals with polysensitization showed significantly higher irritation responses to SLS 1% and 1.5% as assessed by TEWL. /The study/ found an enhanced skin irritation response to be a risk factor for the development of polysensitization to fragrance chemicals. The barrier perturbation pattern and molecular markers of inflammation upon tandem repeated irritation in chronologically aged skin have not been previously studied. /The study/ aimed to investigate the barrier impairment kinetic and in vivo cytokine profile following sequential irritation with sodium lauryl sulfate (SLS) and undiluted toluene (Tol) in aged compared with young skin. Four fields on the volar forearm of healthy aged and young volunteers (median age, respectively, 63.9 and 32.6 years) were sequentially exposed to 0.5% SLS and undiluted toluene in a controlled tandem repeated irritation test; an adjacent nontreated field served as control. The permeability barrier function was monitored by repeated measurements of transepidermal water loss (TEWL), capacitance and erythema every 24 hr up to 96 hr. The stratum corneum cytokines were harvested by sequential tape stripping and quantified by multiplex bead array and enzyme-linked immunosorbent assay. Compared with young skin, aged skin was characterized by delayed and/or less pronounced alterations in the visual irritation score, TEWL, chromametry a*-value and capacitance, assessed by the respective delta-values for each parameter and monitoring time point. In both groups, exposure to SLS/SLS, SLS/Tol and Tol/SLS resulted in decreased interleukin (IL)-1alpha levels, whereas the application of Tol/Tol induced an increase in IL-1alpha. Furthermore, decreased IL-1 receptor antagonist (IL-1RA) levels and a lower IL-1RA/IL-1alpha ratio were found following repeated exposure to the irritants. Our results provide evidence for selective alterations in the cytokine profile and distinct barrier impairment kinetic following tandem repeated irritation with SLS and Tol in aged compared with young skin in vivo. The influence of sodium lauryl sulfate (SLS) on the efficacies of topical gel formulations of foscarnet against herpes simplex virus type 1 (HSV-1) cutaneous infection has been evaluated in mice. A single application of the gel formulation containing 3% foscarnet given 24 hr postinfection exerted only a modest effect on the development of herpetic skin lesions. Of prime interest, the addition of 5% SLS to this gel formulation markedly reduced the mean lesion score. The improved efficacy of the foscarnet formulation containing SLS could be attributed to an increased penetration of the antiviral agent into the epidermis. In vitro, SLS decreased in a concentration-dependent manner the infectivities of herpes viruses for Vero cells. SLS also inhibited the HSV-1 strain F-induced cytopathic effect. Combinations of foscarnet and SLS resulted in subsynergistic to subantagonistic effects, depending on the concentration used. Foscarnet in phosphate-buffered saline decreased in a dose-dependent manner the viability of cultured human skin fibroblasts. This toxic effect was markedly decreased when foscarnet was incorporated into the polymer matrix. The presence of SLS in the gel formulations did not alter the viabilities of these cells. The use of gel formulations containing foscarnet and SLS could represent an attractive approach to the treatment of herpetic mucocutaneous lesions, especially those caused by acyclovir-resistant strains. The mechanisms of herpes simplex virus (HSV) inactivation by sodium lauryl sulfate (SLS) and n-lauroylsarcosine (LS), two anionic surfactants with protein denaturant potency, have been evaluated in cultured cells. Results showed that pretreatment of HSV type 1 (HSV-1) strain F and HSV-2 strain 333 with either surfactant inhibited, in a concentration- and time-dependent manner, their infectivities on Vero cells. SLS was a more potent inhibitor of HSV-2 strain 333 infectivity than LS with respect to the concentration (4.8-fold lower) and time (2.4-fold shorter) required to completely inactivate the virus. No inhibition of both herpes virus strains infectivities was observed when Vero cells were pretreated with either surfactant. LS prevented the binding of HSV-2 strain 333 to cells without affecting the stable attachment and the rate of penetration into cells, whereas SLS exerted the opposite effect. Both SLS and LS inhibited, in a concentration-dependent manner, the HSV-2 strain 333-induced cytopathic effect, probably by affecting newly synthesized virions that come into contact with surfactant molecules present in culture medium. The pretreatment of HSV-2 strain 333 with specific combinations of SLS and LS concentrations inhibited the viral infectivity in a synergistic manner and resulted in only a small increase in their toxicities for exponentially growing Vero cells compared with that caused by each compound alone. Taken together, these results suggest that SLS and LS, alone or combined, could represent potent candidates as microbicides in topical vaginal formulations to prevent the transmission of herpes and possibly other pathogens that cause sexually transmitted diseases, including human immunodeficiency virus type 1. For more Interactions (Complete) data for SODIUM LAURYL SULFATE (6 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat oral 1288 mg/kg LD50 Rat ip 210 mg/kg LD50 Rat iv 118 mg/kg LD50 Mouse ip 250 mg/kg LD50 Mouse iv 118 mg/kg |
|---|---|
| 参考文献 | |
| 其他信息 |
Dodecyl sulfate, [sodium salt] appears as white to pale yellow paste or liquid with a mild odor. Sinks and mixes with water. (USCG, 1999)
Sodium dodecyl sulfate is an organic sodium salt that is the sodium salt of dodecyl hydrogen sulfate. It has a role as a detergent and a protein denaturant. It contains a dodecyl sulfate. Sodium Lauryl Sulfate (SLS) is an anionic surfactant naturally derived from coconut and/or palm kernel oil. It usually consists of a mixture of sodium alkyl sulfates, mainly the lauryl. SLS lowers surface tension of aqueous solutions and is used as fat emulsifier, wetting agent, and detergent in cosmetics, pharmaceuticals and toothpastes. It is also used in creams and pastes to properly disperse the ingredients and as research tool in protein biochemistry. SLS also has some microbicidal activity. An anionic surfactant, usually a mixture of sodium alkyl sulfates, mainly the lauryl; lowers surface tension of aqueous solutions; used as fat emulsifier, wetting agent, detergent in cosmetics, pharmaceuticals and toothpastes; also as research tool in protein biochemistry. Drug Indication SLS is used as a surfactant in shampoos and toothpastes. SLS also has microbicidal activities against both enveloped (Herpes simplex viruses, HIV-1, Semliki Forest virus) and nonenveloped (papillomaviruses, reovirus, rotavirus and poliovirus) viruses, although it has not been approved for this use. Mechanism of Action Like other surfactants, SLS is amphiphilic. It thus migrates to the surface of liquids, where its alignment and aggregation with other SLS molecules lowers the surface tension. This allows for easier spreading and mixing of the liquid. SLS has potent protein denaturing activity and inhibits the infectivity of viruses by by solubilizing the viral envelope and/or by denaturing envelope and/or capsid proteins. Therapeutic Uses /VET/ foot & mouth disease virus is highly resistant to.../sodium lauryl sulfate/, yet TGE virus is sensitive... fungistatic (incl Candida & Trichophyton spp) & concn of 2% & over eliminated drug resistance & sex transfer factors in E coli. Inhibits growth of many G-pos bacteria...ineffective against G-neg types. /VET/ Sodium lauryl sulfate is used as a flea and tick repellant in one registered pesticide product--a flea and tick shampoo for cats and dogs. Sodium lauryl sulfate also is a widely used component of many nonpesticidal consumer products currently marketed in the United States, including shampoos and fruit juices. /VET/ as wetting agent for some antibiotics & antimicrobials (tylosin, sulfaquinoxaline, tyrothricin, etc) for oral & topical use. Widely used in ointment bases & as wetting agent for some insecticides & anthelmintics. Also useful in producing clear gel shampoos. /EXPL THER/ About 1/3 of HIV positive mothers transmit the virus to their newborns, and 1/2 of these infections occur during breastfeeding. Sodium dodecyl sulfate (SDS), an anionic surfactant, is a common ingredient of cosmetic and personal care products. SDS is "readily biodegradable" with low toxicity and "is of no concern with respect to human health". Up to 1 g of SDS/kg is the maximum safe dose for children. Alkyl sulfates, including SDS, are microbicidal against HIV types 1 and 2, herpes simplex virus type 2 (HSV-2), human papillomaviruses and chlamydia. /The study/ hypothesizes that SDS treatment of milk will inactivate HIV-1 without significant harm to its nutritional value and protective functions and may define a treatment of choice for breastwas at 37 degrees C for 10 min. SDS-PAGE and Lowry were used to analyze protein content. Antibody content and function was studied by rocket immunoelectrophoresis (RIE), immunoturbodimentric (ITM) quantitation and ELISA. The creamatocrit was also analyzed. HIV-1 infectivity was measured by MAGI assay. SDS removal was by Detergent-OutN (Geno Technology, Inc.). SDS quantitation is by methylene blue-chloroform method. Inactivation of HIV-1 with SDS occurs at or above 0.025%. In milk samples, 1% and 0.1% SDS reduced HSV-2 infectivity. At least 90% of SDS can be efficiently removed with Detergent-OutN, with protein recovery of 80%-100%. Gross protein species are conserved as indicated by PAGE analyses. Fat and energy content of SDS-treated breast milk remains unchanged. 0.1% SDS can be removed from human milk without altering the creamatocrit. ELISA of serum IgG (rubella) proved it remains functional in the presence of SDS and after its removal. sIgA, IgG and IgM in breast milk are conserved after SDS-treatment when measured by RIE and ITM. CONCLUSIONS: SDS (0.025%) can inactivate HIV-1 in vitro and HSV-2 in breast milk. SDS can be efficiently removed from milk samples. SDS treatment of milk does not significantly alter protein content. Antibody function in serum and levels in breast milk are maintained after treatment and removal of SDS. 0.1% SDS does not alter fat concentration in milk and energy content is conserved. SDS or related compounds may be used to prevent breast milk transmission of HIV-1. /EXPL THER/ A broad-spectrum vaginal microbicide must be effective against a variety of sexually transmitted disease pathogens and be minimally toxic to the cell types found within the vaginal epithelium, including vaginal keratinocytes. /The study/ assessed the sensitivity of primary human vaginal keratinocytes to potential topical vaginal microbicides nonoxynol-9 (N-9), C31G, and sodium dodecyl sulfate (SDS). Direct immunofluorescence and fluorescence-activated cell sorting analyses demonstrated that primary vaginal keratinocytes expressed epithelial cell-specific keratin proteins. Experiments that compared vaginal keratinocyte sensitivity to each agent during a continuous, 48-hr exposure demonstrated that primary vaginal keratinocytes were almost five times more sensitive to N-9 than to either C31G or SDS. To evaluate the effect of multiple microbicide exposures on cell viability, primary vaginal keratinocytes were exposed to N-9, C31G, or SDS three times during a 78-hr period. In these experiments, cells were considerably more sensitive to C31G than to N-9 or SDS at lower concentrations within the range tested. When agent concentrations were chosen to result in an endpoint of 25% viability after three daily exposures, each exposure decreased cell viability at the same constant rate. When time-dependent sensitivity during a continuous 48-hr exposure was examined, exposure to C31G for 18 hr resulted in losses in cell viability not caused by either N-9 or SDS until at least 24 to 48 hr. Cumulatively, these results reveal important variations in time- and concentration-dependent sensitivity to N-9, C31G, or SDS within populations of primary human vaginal keratinocytes cultured in vitro. These investigations represent initial steps toward both in vitro modeling of the vaginal microenvironment and studies of factors that impact the in vivo efficacy of vaginal topical microbicides. Pharmacodynamics SLS is an anionic surfactant. Its amphiphilic properties make it an ideal detergent. |
| 分子式 |
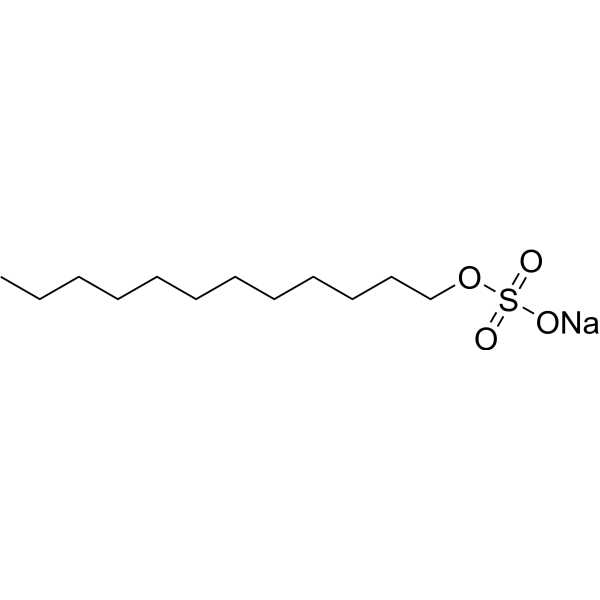
C12H25NAO4S
|
|---|---|
| 分子量 |
288.37
|
| 精确质量 |
288.137
|
| CAS号 |
151-21-3
|
| 相关CAS号 |
Sodium-dodecyl sulfate-d25;110863-24-6
|
| PubChem CID |
3423265
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
0.25g/ml
|
| 熔点 |
206ºC
|
| 闪点 |
>100ºC
|
| LogP |
4.464
|
| tPSA |
74.81
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
12
|
| 重原子数目 |
18
|
| 分子复杂度/Complexity |
249
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
DBMJMQXJHONAFJ-UHFFFAOYSA-M
|
| InChi Code |
InChI=1S/C12H26O4S.Na/c1-2-3-4-5-6-7-8-9-10-11-12-16-17(13,14)15;/h2-12H2,1H3,(H,13,14,15);/q;+1/p-1
|
| 化学名 |
sodium;dodecyl sulfate
|
| 别名 |
NSC-402488; NSC 402488; Sodium lauryl sulfate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~250 mg/mL (~866.91 mM)
H2O : ~100 mg/mL (~346.76 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.67 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (8.67 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.4678 mL | 17.3388 mL | 34.6777 mL | |
| 5 mM | 0.6936 mL | 3.4678 mL | 6.9355 mL | |
| 10 mM | 0.3468 mL | 1.7339 mL | 3.4678 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。