| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following once-daily administration of 50 to 200 mg for two weeks, the mean peak plasma concentrations (Cmax) of sertraline occurred between 4.5 to 8.4 hours after administration, and measured at 20 to 55 μg/L. Steady-state concentrations are reached after 1 week following once-daily administration, and vary greatly depending on the patient. Bioavailability has been estimated to be above 44%. The area under the curve in healthy volunteers after a 100mg dose of sertraline was 456 μg × h/mL in one study. **Effects of food on absorption** The effects of food on the bioavailability of the sertraline tablet and oral concentrate were studied in subjects given a single dose with and without food. For the tablet, AUC was slightly increased when sertraline was administered with food, the Cmax was 25% greater, and the time to peak plasma concentration was shortened by about 2.5 hours. For the oral concentrate preparation of sertraline, peak concentration was prolonged by approximately 1 hour with the ingestion of food. Since sertraline is extensively metabolized, excretion of unchanged drug in the urine is a minor route of elimination, with 12-14% of unchanged sertraline excreted in the feces. Sertraline is widely distributed, and its volume of distribution is estimated to be more than 20L/kg. Post-mortem studies in humans have measured liver tissue concentrations of 3.9–20 mg/kg for sertraline and between 1.4 to 11 mg/kg for its active metabolite, N-desmethyl-sertraline (DMS). Studies have also determined sertraline distributes into the brain, plasma, and serum. In pharmacokinetic studies, the clearance of a 200mg dose of sertraline in studies of both young and elderly patients ranged between 1.09 ± 0.38 L/h/kg - 1.35 ± 0.67 L/h/kg. GI absorption: >or= 44%; time to reach peak plasma concn: 6-8 hr; oral clearance (single dose): 96 L/hr; protein binding: 99%; urinary excretion (radioactivity): 44% of oral dose; fecal excretion (radioactivity): 44% of oral dose. /from table/ Sertraline is absorbed readily through the GI tract. Its absolute bioavailability has not been determined in humans. It displays first-order kinetics. Max plasma concns following doses of 50 & 200 mg are 22-29 ug/L (ng/ml). These concns are reached in 4.5-8.4 hr. Serum levels at steady state are 10-120 ng/mL of sertraline & its desmethyl metabolites. Plasma protein binding is extensive (approx 98%) to both albumin & alpha1-acid glycoprotein. At concns up to 300 & 200 ug/mL, respectively, sertraline & N-desmethyl-sertraline do not appear to alter the plasma protein binding of two other highly protein-bound drugs, warfarin & propranolol. Distribution following oral admin of sertraline is biphasic with a prolonged absorption phase. The elimination phase begins 12-16 hr following the dose. The volume of distribution has not been determined in humans but is more than 20 L/kg in rats & dogs. Both sertraline & its metabolites exhibit extensive distribution into tissues outside the blood. ... The elimination half-life (beta) of sertraline in humans is 24-25 hr. The clinically active desmethyl metabolite is eliminated more slowly than the parent drug with a half-life of approx 66 hr. Unchanged sertraline is not detected in the urine. Slow but consistent. Bioavailablity and absorption rate are increased if sertraline is taken with food. Both sertraline and its metabolites are extensively distributed into tissues. In animal studies, the volume of distribution (volD) exceeded 20 L/kg. Metabolism / Metabolites Sertraline is heavily metabolized in the liver and has one major active metabolite. It undergoes N-demethylation to form N-desmethylsertraline, which is much less potent in its pharmacological activity than sertraline. In addition to N-demethylation, sertraline metabolism involves N-hydroxylation, oxidative deamination, and finally, glucuronidation. The metabolism of sertraline is mainly catalyzed by CYP3A4 and CYP2B6, with some activity accounted for by CYP2C19 and CYP2D6. Sertraline undergoes extensive metabolism. The parent drug is N-demethylated, followed by glucuronidation, deamination, or both. Most metabolites in the urine are alpha-hydroxy-ketone glucuronides. Depression is one of the most common psychiatric disorders. A variety of different chemical structures have been found to have antidepressant activity. The number is constantly growing; however, as yet, no one group has been found to have a clear therapeutic advantage over the others. The major indication for antidepressant drugs is depression, but a number of side effects have been established by clinical experience and controlled trials. It is clear that, to some extent, any drug or chemical substance administered to the mother is able to cross the placenta unless it is destroyed or altered during metabolism. Placental transport of maternal substrates to the fetus and of substances from the fetus to the mother is established at about the fifth week of fetal life. Traditionally, teratogenic effects of antidepressants or other drugs have been noted as anatomic malformation. It is clear that these are dose- and time-related and that the fetus is at great risk during the first 3 months of gestation. However, it is possible for antidepressants to exert their effects on the fetus at other times during pregnancy as well as to infants during lactation. Administration of antidepressants to pregnant women presents a unique problem for the physician. Not only must maternal pharmacologic mechanisms be taken into consideration when prescribing an antidepressant drug, but the fetus must also be regarded as a potential recipient of the drug. Certain results are evident with regard to drugs administered during lactation. It is essential that physicians need to be aware of the results of animal studies in this area and of the potential risk of maternal drug ingestion to the suckling infant. Sertraline has known human metabolites that include N-desmethylsertraline. Extensively metabolized in the liver. Sertraline metabolism involves N-demethylation, N-hydroxylation, oxidative deamination, and glucuronidation of sertraline carbamic acid. Sertraline undergoes N-demethylation primarily catalyzed by cytochrome P450 (CYP) 2B6, with CYP2C19, CYP3A4 and CYP2D6 contributing to a lesser extent. Deamination occurs via CYP3A4 and CYP2C19. In vitro studies have shown that monoamine oxidase A and B may also catalyze sertraline deamination. Sertraline N-carbamoyl glucuronidation has also been observed in human liver microsomes. Route of Elimination: Sertraline is extensively metabolized and excretion of unchanged drug in urine is a minor route of elimination. Half Life: The elimination half-life of sertraline is approximately 25-26 hours. The elimination half-life of desmethylsertraline is approximately 62-104 hours. Biological Half-Life The elimination half-life of sertraline is approximately 26 hours. One reference mentions an elimination half-life ranging from 22-36 hours. Elimination half-life, parent (metabolite): 24 (65) hours. /from table/ Elimination half-life: 24 to 26 hours |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION: Sertraline is a selective serotonin reuptake inhibitor antidepressant agent. Sertraline hydrochloride is a white solid crystal or powder. It is soluble in water and slightly soluble in isopropyl alcohol. Indications: Psychoanaleptic Antidepressant and bicyclic antidepressant Accepted: Major mental depression and prevention of relapse and recurrence of depression. HUMAN EXPOSURE: Main risks and target organs: Sertraline is a selective serotonin reuptake inhibitor (SSRI). When taken alone it is safer in overdose than most other classes of antidepressants. Patients who ingest a sertraline overdose generally experience only mild neurological and gastroenterological symptoms; significant cardiovascular toxicity is unusual. The serotonergic effects of sertraline may be enhanced when sertraline is combined with tricyclic antidepressants, monoamine oxidase inhibitors (MAOIs), carbamazepine, lithium or serotonergic substances. A life threatening serotonin syndrome consisting of hyperthermia, tremor and convulsions can develop when sertraline is ingested with these drugs. Summary of clinical effects: Lightheadedness, drowsiness, tremor in upper extremities; nausea, vomiting, diarrhea. Diagnosis: Diagnosis of sertraline poisoning is clinical and based on history of overdose and/or access to sertraline and the presence of minor neurological and/or gastroenterological symptoms. Co-ingestion of tricyclic antidepressants and/or MAOIs should be suspected and the diagnosis of the serotonin syndrome should be considered in the presence of three or more of the following symptoms: behavioral change (confusion or hypomania), agitation, myoclonus, ocular clonus, sustained ankle clonus, hyperreflexia, sweating, shivering, tremor, diarrhea, motor incoordination, muscle rigidity, fever. The differential diagnoses include neuroleptic malignant syndrome, acute poisoning with strychnine, acute sepsis, or severe metabolic disturbance. Contraindications: Absolute: Hypersensitivity to sertraline. Children less than 15 years old. Co-administration of sumatriptan, non selective monoamine oxidase inhibitor (MAOI) and MAOI B-selective antidepressants. Relative: Co-administration of MAOI A-selective antidepressants. Pregnancy and breast feeding. Routes of exposure: Oral: Sertraline is available as capsules, thus ingestion is the most common route of exposure. Kinetics: Absorption by route of exposure: Sertraline is slowly and completely absorbed from the gastrointestinal tract. Peak plasma concentrations (Cmax) occur between 4.5 and 8.5 hours after ingestion of a single dose. The presence of food slightly increases sertraline bioavailability and Cmax increases by 25%. Sertraline undergoes extensive first pass metabolism in the liver. Distribution by route of exposure: Widely distributed throughout body tissues and highly bound to plasma proteins (about 98 %). The apparent volume of distribution is 20 L/kg. The plasma sertraline level was reported to be 20 to 48 ug/L after at least 1 week of treatment with 100 mg sertraline daily, and it ranged from 40 to 187 ug/L after 200 mg. Plasma sertraline concentrations increase proportionally to the administered dose, unlike fluoxetine and paroxetine. Cmax and area under the plasma concentration-time curve values are increased, and elimination half-life is prolonged in elderly patients but these changes do not appear to warrant dose adjustment in this patient group. Biological half-life by route of exposure: After oral doses, plasma half-life is 24 to 26 hours. Metabolism: Sertraline is extensively metabolized in the liver to N-desmethylsertraline, whose half-life is 2 to 3 times longer than sertraline. N-desmethylsertraline is 10 times less active as an inhibitor of serotonin re-uptake in vitro, and has almost no activity in animal models. Elimination and excretion: N-desmethylsertraline is oxidatively deaminated to desmethylsertraline ketone which, in turn, undergoes hydroxylation to an alpha-hydroxyketone and alcohol; these metabolites are then conjugated and excreted in equal amounts in the urine and feces; a small amount of unchanged drug (less than 0.2 %) is excreted in the urine. There are few data about the excretion of sertraline and its metabolites in breast milk did not detect sertraline in the serum of an infant exclusively breastfed by his mother, after 3 weeks and 7 weeks of treatment, although sertraline could be detected in breast milk. Pharmacology and toxicology: Mode of action: Toxicodynamics: Sertraline is a potent inhibitor of serotonin re-uptake by central nervous system neurones and may interact with other drugs or circumstances which cause serotonin release. The enhancement of the serotonergic effects may produce a life-threatening serotonin syndrome. Sertraline, like the other SSRIs fluoxetine and paroxetine, can inhibit in vivo and in vitro, the hepatic isoenzyme 2D6 of the cytochrome P450 system (CYP2D6), which is involved in the oxidative metabolism of numerous drugs. Caution should be used when combining sertraline with CYP2D6 substrates (desipramine, nortriptyline, haloperidol, thioridazine, flecainide, codeine, propranolol, metoprolol), as sertraline can cause a significant increase in the serum concentrations of these drugs. In vitro studies suggest that sertraline may be a substrate for, but does not inhibit another hepatic iso-enzyme of the cytochrome P450 system, CYP3A3/4, which is involved in the metabolism of carbamazepine. A study performed in healthy volunteers showed no evidence of a pharmacodynamic drug-drug interaction between sertraline and carbamazepine. Pharmacodynamics: Sertraline specifically inhibits central nervous system neuronal re-uptake of serotonin, thus increasing the concentration of the serotonin at the synapse and enhancing of serotonergic neuronal transmission. The increased availability of serotonin is thought to be linked with the improvement in depression accounted for by sertraline treatment. Sertraline has no direct effect on the re-uptake of noradrenaline, dopamine or GABA. Unlike most tricyclic antidepressants, it has no significant affinity for alpha1-adrenergic, H1-histamine, and muscarinic receptors. Furthermore, sertraline does not show significant affinity for D1 and D2 dopaminergic, alpha2 and adrenergic, benzodiazepine and opioid receptors. The selectivity of sertraline may account for the lower incidence of some adverse effects such as sedation, orthostatic hypotension and anticholinergic effects. Like tricyclic antidepressants, MAOIs, and other SSRIs, sertraline significantly reduces REM (rapid eye movement) sleep density, REM time and the REM percentage of total sleep time in patients with major depression. Adults: Overdoses up to 4500 mg of sertraline alone produced mild drowsiness and serious toxicity did not develop in the 48 patients. Children: In a case series of pediatric overdoses, sertraline caused no symptoms in 10 children less than 5 year old; eight of these received gastrointestinal decontamination. Interactions: Coadministration of drugs increasing the level of serotonin: tricyclic antidepressants, other SSRIs, MAOIs, reversible inhibitors of monoamine oxidase (RIMAs), lithium, may cause a serotonin syndrome. At least 2 weeks should elapse after discontinuing a MAOI before starting therapy with sertraline. Sertraline should be stopped at least 1 week before beginning MAOI therapy. Sertraline should not be concomitantly used with sumatriptan, which is a selective agonist at serotonin type 1D receptors, because of possible hypertensive crises and severe coronary vasoconstriction, and advises a washout period of 1 week after cessation of sertraline. A clinical study involving 103 episodes of migraine in patients taking any SSRIs, did not show evidence of significant adverse effects. Cimetidine inhibits the metabolism of sertraline, leading to increased plasma concentrations; close clinical monitoring and/or reduced sertraline doses are recommended. By extrapolation from data available for fluoxetine, drug interactions with oral anticoagulants and carbamazepine might occur, though in vitro and in vivo studies performed with carbamazepine did not show evidence of interactions and though no cases have been reported to date. Treatment with sertraline was associated with worsening and/or recurrence of the lysergide (LSD) flashbacks in two adolescents with a long history of polydrug abuse. They had stopped taking LSD 10 months before sertraline therapy. Main adverse effects: The most common adverse effects reported with therapeutic doses of sertraline are primarily nausea, diarrhea, dyspepsia, dry mouth, insomnia, somnolence, tremor, dizziness, headache and male sexual dysfunction (delayed ejaculation). These adverse effects are reported to occur in 10 to 20 % of patients, and they cause patients to stop therapy in approximately 1 to 4 % of cases. Manic episodes may be provoked in patients with bipolar disorders. If this occurs, sertraline should be discontinued and a sedative antipsychotic drug should be administered. Less common adverse effects include, pruritus, alopecia, and extrapyramidal symptoms. Several cases of hyponatremia and the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) have been reported, mainly in elderly patients. Galactorrhea developed in a 40 year old woman receiving sertraline 150 mg/day during 11 week of dosing. Several cases of stuttering have been described. Bruxism causing significant physical consequences was associated with sertraline and other SSRIs in a case series reported. A case of anisocoria was reported. Increase in serum AST and ALT levels has occurred, and returned to normal after treatment was stopped. Sertraline caused prolonged bleeding time in one patient; agranulocytosis was also reported. ANIMAL/PLANT STUDIES: Symptomatology: decreased food intake, hyperactivity, muscular weakness, convulsions. Chronic toxicity: oral doses of serataline for 6 and 12 months; several adverse effects occurred during the first weeks, including hypersalivation, abnormal movements of the head, disorientation, agitation; resulted in convulsions in 2 dogs; all of these effects where temporary and resolved spontaneously despite continuation of sertraline administration. An increase of liver weight associated with a rise in plasma alkaline phosphatase enzymes was noted, due to the properties of enzyme induction of sertraline. Carcinogenicity: Animal studies: In rats a slight increase in the number of follicular and thyroid adenomas was observed in relation with hepatic enzyme induction; these findings cannot be extrapolated to man, because of species differences in the metabolic mechanisms. Teratogenicity: Animal studies: Fertility in rats was not affected. Sertraline did not show embryotoxic or teratogenic properties in rat and rabbit models. However, in the rat, sertraline caused a delay in fetal ossification and a delay in apparition of teeth in the young. A decrease in food intake inducing a delay in growth in the young, proportional to the administered dose, sometimes associated with hyperactivity, was also observed. Mutagenicity: In vitro and in vivo: sertraline did not show mutagenicity for chromosomes and genes. The exact mechanism of action sertraline is not fully known, but the drug appears to selectively inhibit the reuptake of serotonin at the presynaptic membrane. This results in an increased synaptic concentration of serotonin in the CNS, which leads to numerous functional changes associated with enhanced serotonergic neurotransmission. It is suggested that these modifications are responsible for the antidepressant action observed during long term administration of antidepressants. It has also been hypothesized that obsessive-compulsive disorder is caused by the dysregulation of serotonin, as it is treated by sertraline, and the drug corrects this imbalance. Interactions Examples of drug interactions with serotonin-reuptake inhibitors include potentiation of agents metabolized prominently by CYP1A2 (e.g. beta-adrenergic receptor antagonists, caffeine, several antipsychotic agents, & most tricyclic antidepressants); CYP2C9 (carbamazepine); CYP2C19 (barbiturates, imipramine, propranolol, phenyltoin); CYP2D6 (beta-adrenergic receptor antagonists, some antipsychotics, many antidepressants); CYP3A3/4 (benzodiazepines, carbamazepine, many antidepressants, & several antibiotics). /Serotonin-reuptake inhibitors/ Antidepressants potentiate the effects of alcohol & probably other sedatives. The anticholinergic activity of tricyclic antidepressants can add to that of antiparkinsonism agents, antipsychotic drugs of low potency (especially clozapine & thioridazine), or other compounds with antimuscarinic activity to produce toxic effects. Tricyclic antidepressants have prominent & potentially dangerous interactions with biogenic amines, such as norepinephrine, which normally are removed from their site of action by neuronal uptake. However, drugs that inhibit norepinephrine transport also block the effects of indirectly acting amines, such as tyramine, which must be taken up by sympathetic neurons to release norepinephrine. Presumable by a similar mechanism, tricyclic antidepressants prevent the antihypertensive action of adrenergic neuron blocking agents such as guanadrel. Tricyclic agents & trazodone also can block the centrally mediated antihypertensive action of clonidine. /Antidepressants/ Serotonin-reuptake inhibitors & virtually any agent with serotonin-potentiating activity can interact dangerously or even fatally with MAO inhibitors (particularly long-acting MAO inhibitors). ... The resulting reactions have been referred to as "serotonin syndrome". This syndrome typically includes akasthisia-like restlessness, muscle twitches & myoclonus, hyperreflexia, sweating, penile erection, shivering, & tremor as a prelude to more severe intoxication, with seizures & coma. The reaction is often self-limiting if the diagnosis is made quickly & the offending agents are discontinued. The precise pathophysiological mechanisms underlying these toxic syndromes remain ill-defined. Newer MAO inhibitors (e.g. selegiline, moclobemide) also should be considered to have some risk of such interactions. /Serotonin-reuptake inhibitors/ Because of its extensive protein binding. Sertraline may cause a shift in plasma concns of other drugs similarly tightly protein bound (eg, warfarin, propranolol). the risk of using sertraline in combination with other CNS adrenergic drugs has not been systematically evaluated. Sertraline appears to induce hepatic microsomal enzymes. Patients receiving a sertraline reuptake inhibitor drug in combination with a monoamine oxidase inhibitor may develop serious, sometimes fatal, reactions including hyperthermia, rigidity, myoclonus, autonomic instability, & mental changes (extreme agitation, delirium, coma). At least 14 days should be allowed after starting sertraline before starting a MAOI. For more Interactions (Complete) data for SERTRALINE (7 total), please visit the HSDB record page. |
| 参考文献 |
J Pharmacol Exp Ther.1983 Sep;226(3):686-700.
|
| 其他信息 |
Therapeutic Uses
Antidepressants, especially serotonin-reuptake inhibitors, also are employed in the management of post-traumatic stress disorder, marked by anxiety, startle, painful recollection of the traumatic events, & disturbed sleep. ... The serotonin-reuptake inhibitors are agents of choice in obsessive-compulsive disorder, as well as in possibly related syndromes of impulse dyscontrol or obsessive preoccupations, including compulsive habits, bulimia (but usually not anorexia) nervosa, & body dysmorphic disorder. While their benefits may be limited, serotonin-reuptake inhibitors offer an important advance in the medical treatment of these often chronic & sometimes incapacitating disorders for which no other medical treatment, by itself, has been consistently effective, the effectiveness of pharmacological treatment of these commonly treatment-resistant disorders is greatly enhanced by use of behavioral treatments. In addition to the wide use of modern antidepressants to treat depression commonly associated with general medical illnesses, several psychosomatic disorders may respond at least partly to treatment with antidepressants of the tricyclic, MAO inhibitor, or serotonin-reuptake inhibitor types. These include chronic pain disorders, including adiabetic & other peripheral neuropathic syndromes (for which tertiaryamine tricyclics are probably superior to fluoxetine); fibromyalgia; peptic ulcer & irritable bowel syndrome; chronic fatigue; cataplexy; tics; migraine; & sleep apnea. /Antidepressants; Serotonin-reuptake inhibitors/ Sertraline is indicated for the treatment of major depressive disorder. Treatment of acute depressive episodes typically requires 6 to 12 months of antidepressant therapy. Patients with recurrent or chronic depression may require long-term treatment. Sertraline showed effective maintenance of antidepressant response for up to 52 weeks of treatment in a placebo-controlled trial. /Included in US product labeling/ Sertraline is indicated for the treatment of obsession and compulsions in adults and children 6 years of age and older with obsessive-compulsive disorder. /Included in US product labeling/ Sertraline is indicated for the treatment of panic disorder with or without agoraphobia. /Included in US product labeling/ Drug Warnings Side effects of Sertraline include: mild agitation, minimal sedation, moderately severe GI effects, & moderately severe sexual effects. /from table/ Sertraline has been tested in children 6 to 17 years of age and, in effective doses, has not been shown to cause different side effects or problems than it does in adults. However, the effects of long-term use of sertraline on the growth, development, and maturation of children and adolescents are unknown. Because of the anorectic effect of sertraline, body weight and growth should be monitored in children receiving long-term treatment. No geriatrics-specific problems have been documented in studies done to date that include elderly patients. However, in one study, clearance of sertraline in 16 elderly patients was about 40% lower than clearance in a group of younger subjects, indicating that the steady-state will take 2 to 3 weeks to achieve in elderly patients. A reduced initial dosage is recommended in elderly patients. In a single-dose study, mean elimination half-life of sertraline was prolonged from 22 hours in healthy subjects to 52 hours in patients with mild, stable cirrhosis; peak concentrations and AUC were increased 1.7 and 4.4 times, respectively, in patients with hepatic impairment; decreased dosage or less frequent dosing is recommended. For more Drug Warnings (Complete) data for SERTRALINE (15 total), please visit the HSDB record page. Pharmacodynamics Sertraline improves or relieves the symptoms of depression, OCD, post-traumatic stress disorder, obsessive-compulsive disorder, panic disorder, and premenstrual dysphoric disorder via the inhibition of serotonin reuptake. Clinical studies have shown that it improves cognition in depressed patients. It has less sedative, anticholinergic, and cardiovascular effects than the tricyclic antidepressant drugs because it does not exert significant anticholinergic, antihistamine, or adrenergic (alpha1, alpha2, beta) blocking activity. The onset of action and beneficial effects are usually noticed after 4-6 weeks, for reasons that are not fully understood and currently under investigation. |
| 分子式 |
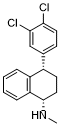
C17H17CL2N
|
|---|---|
| 分子量 |
306.23
|
| 精确质量 |
305.073
|
| CAS号 |
79617-96-2
|
| 相关CAS号 |
79559-97-0 (HCl);79617-96-2;
|
| PubChem CID |
68617
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
416.3±45.0 °C at 760 mmHg
|
| 熔点 |
188-190 °C(lit.)
|
| 闪点 |
205.6±28.7 °C
|
| 蒸汽压 |
0.0±1.0 mmHg at 25°C
|
| 折射率 |
1.621
|
| LogP |
4.81
|
| tPSA |
12.03
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
20
|
| 分子复杂度/Complexity |
322
|
| 定义原子立体中心数目 |
2
|
| SMILES |
CN[C@H]1CC[C@@H](C2=CC(=C(C=C2)Cl)Cl)C3=CC=CC=C31
|
| InChi Key |
VGKDLMBJGBXTGI-SJCJKPOMSA-N
|
| InChi Code |
InChI=1S/C17H17Cl2N/c1-20-17-9-7-12(13-4-2-3-5-14(13)17)11-6-8-15(18)16(19)10-11/h2-6,8,10,12,17,20H,7,9H2,1H3/t12-,17-/m0/s1
|
| 化学名 |
(1S,4S)-4-(3,4-dichlorophenyl)-N-methyl-1,2,3,4-tetrahydronaphthalen-1-amine
|
| 别名 |
HSDB7037 HSDB-7037Sertraline Free Base HSDB 7037
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.2655 mL | 16.3276 mL | 32.6552 mL | |
| 5 mM | 0.6531 mL | 3.2655 mL | 6.5310 mL | |
| 10 mM | 0.3266 mL | 1.6328 mL | 3.2655 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。