| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| Other Sizes |
|
| 靶点 |
HDAC1 ( IC50 = 36 nM ); HDAC2 ( IC50 = 47 nM ); HDAC4 ( IC50 = 510 nM ); HDAC6 ( IC50 = 14000 nM )
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:与TSA不同,罗米地辛的活性形式redFK强烈抑制HDAC1和HDAC2,IC50分别为1.6 nM和3.9 nM,但抑制HDAC4和HDAC6相对较弱,IC50分别为25 nM和790 nM。 Romidepsin 对这些 HDAC 的抑制作用比 redFK 弱 17-23 倍,IC50 分别为 36 nM、47 nM、510 nM 和 14 μM。 Romidepsin 在 HeLa 细胞中的处理可诱导组蛋白乙酰化和 p21 表达,EC50 为 3.0 nM,由于 redFK 的不稳定性,其 EC50 为 11 nM,比 redFK 更强。除了 G2/M 停滞之外,Romidepsin 治疗还会导致细胞周期蛋白 D1 下调和 p53 独立的 p21 诱导,从而抑制 CDK 和 Rb 去磷酸化,从而导致 G1 早期生长停滞。罗米地辛抑制 A549 细胞增殖的作用比 TSA 强 100 倍,比丁酸盐强 1,000,000 倍。 Romidepsin 抑制 U-937、K562 和 CCRF-CEM 细胞的生长,IC50 分别为 5.92 nM、8.36 nM 和 6.95 nM。 Romidepsin 在与 H3 和 H4 乙酰化和 HDAC 抑制发生相对应的浓度下促进慢性淋巴细胞白血病 (CLL) 细胞凋亡,选择性涉及 caspase 8 和效应 caspase 3 的激活以及 c-FLIP 蛋白的下调。在 13 种肾细胞癌细胞系中的 11 种 (85%) 和 37 种其他癌细胞系中的 16 种 (43%) 中,罗米地辛治疗上调肿瘤死亡受体,并增强自然杀伤 (NK) 介导的肿瘤杀伤作用。罗米地辛对一组套细胞淋巴瘤 (MCL) 细胞系表现出浓度依赖性细胞毒性 激酶测定:对于酶测定,将 10 μL [3H]乙酰基标记的组蛋白 (25,000 cpm/10 μg) 添加到 90 μL 的在浓度不断增加的罗米地辛存在下,从过表达 HDAC1 或 HDAC2 的 293T 细胞中提取 HDAC 酶组分,并将混合物在 37°C 下孵育 15 分钟。酶反应至少 1 小时呈线性。加入 10 μL 浓 HCl 终止反应。释放出的[3H]乙酸用1mL乙酸乙酯萃取,取0.9mL溶剂层加入5mL计数闪烁液II水溶液中,测定放射性。 IC50值由至少三个独立的剂量反应曲线确定。细胞测定:将细胞在 96 孔板中暴露于不同浓度的罗米地辛 72 小时。将 20 μL 5 mg/mL MTT 溶液的 PBS 添加到每个孔中,持续 4 小时。除去培养基后,向每孔中添加 170 μL DMSO 以溶解甲臜晶体。测定 540 nm 处的吸光度。此外,将细胞与台盼蓝一起孵育,并在血细胞计数器中计数蓝色(死)细胞和透明(活)细胞的数量。对于细胞周期分析,将细胞在含有 0.05 mg/mL 碘化丙啶、1 mM EDTA、0.1% Triton X-100 和 1 mg/mL RNase A 的 PBS 溶液的碘化丙啶染色溶液中孵育 30 分钟。然后使悬浮液通过尼龙网过滤器并在 Becton Dickinson FACScan 上进行分析。
|
| 体内研究 (In Vivo) |
在基质胶塞测定中,罗米地辛治疗可有效抑制鸡胚和成年小鼠的新血管形成。每周两次给予罗米地辛 0.1-1 mg/kg 可显着延长患有 U-937 淋巴瘤的小鼠的生存期,中位生存时间分别为 30.5 天(0.56 mg/kg)和 33 天(0.32 mg/kg)(对比. 对照小鼠 20 天)。
|
| 酶活实验 |
在酶测定中,在浓度不断增加的罗米地辛存在下,从过表达 HDAC1 或 HDAC2 的 293T 细胞中提取的 90 μL HDAC 酶组分与 10 μL [3H]乙酰基标记的组蛋白混合( 25,000 cpm/10 微克)。然后将混合物在 37°C 下孵育 15 分钟。在至少 60 分钟内,酶反应呈线性。加入 10 μL 浓 HCl 终止反应。为了测定放射性,用 1 mL 乙酸乙酯萃取释放的 [3H]乙酸后,将 0.9 mL 溶剂层添加到 5 mL 计数闪烁剂 II 水溶液中。至少使用三个单独的独立剂量反应曲线来计算IC50值。
|
| 细胞实验 |
在 96 孔板中,细胞暴露于不同剂量的罗米地辛,持续 72 小时。在 4 小时内,向每个孔中添加 20 μL 5 mg/mL MTT 的 PBS 溶液。为了溶解甲臜晶体,在除去培养基后向每个孔中添加 170 μL DMSO。在 540 nm 处,计算吸光度。此外,将台盼蓝添加到细胞中,并在血细胞计数器中计数透明(活)和蓝色(死)细胞的数量。将细胞在含有 0.05 mg/mL 碘化丙啶、1 mM EDTA、0.1% Triton X-100 和 1 mg/mL RNase A 的 PBS 溶液的碘化丙啶染色溶液中孵育 30 分钟,以进行细胞周期分析。然后,悬浮液通过尼龙网过滤器后,使用 Becton Dickinson FACScan 进行检查。
|
| 动物实验 |
Male scid mice inoculated i.p. with U-937 cells
~1 mg/kg once or twice a week Treated i.p. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Romidepsin exhibited linear pharmacokinetics at standard doses. 44.5L 8.4L/h Metabolism / Metabolites Romidepsin undergoes extensive hepatic metabolism in vitro primarily by CYP3A4 with minor contribution from CYP3A5, CYP1A1, CYP2B6 and CYP2C19. Biological Half-Life Approximately 3 hours |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In clinical trials of romidepsin in patients with CTLC and PTLC, the rates of serum enzyme elevations during therapy ranged from 7% to 20%, but the abnormalities were usually transient and mild and did not require dose modifications. Serum ALT elevations above 5 times ULN occurred in 6% of patients. In the preregistration clinical trials of romidepsin, there were no reports of hepatitis, jaundice or clinically apparent liver injury among the treated subjects. Romidepsin has had limited clinical use, but there is no evidence that it is associated with significant liver injury. Romidepsin also has immunomodulatory activities and has been reported to cause reactivation of latent DNA viruses including Epstein-Barr, varicella zoster and hepatitis B virus. Reactivation of hepatitis B occurred in a patient who was initially negative for HBsAg, but reactive for anti-HBc and anti-HBs. Nevertheless, the clinical features of hepatitis B reactivation were mild and responded to oral antiviral therapy. In patients with EBV associated lymphoma, romidepsin has been associated with severe reactivation of EBV infection and acute hepatitis that can be severe and even fatal. Likelihood score: C (probable cause of clinically apparent liver injury, which can be due to reactivation of hepatitis B or EBV infection). Protein Binding Highly protein bound in plasma (92%-94%) |
| 参考文献 |
[1]. Cancer Res . 2002 Sep 1;62(17):4916-21. [2]. Br J Cancer . 2000 Sep;83(6):817-25. [3]. Mol Cancer Ther . 2002 Sep;1(11):937-41. [4]. Int J Cancer . 2002 Jan 20;97(3):290-6. [5]. Biochem Pharmacol . 2002 Oct 1;64(7):1079-90. [6]. Blood . 2003 Jul 15;102(2):652-8. [7]. Cancer Res . 2006 Jul 15;66(14):7317-25. |
| 其他信息 |
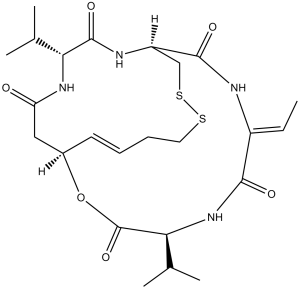
Romidepsin is a cyclodepsipeptide consisting of the cyclic disulfide of (2Z)-2-aminobut-2-enoyl, L-valyl, (3S,4E)-3-hydroxy-7-sulfanylhept-4-enoyl, D-valyl and D-cysteinyl residues coupled in sequence and cyclised head-to tail. It has a role as an antineoplastic agent and an EC 3.5.1.98 (histone deacetylase) inhibitor. It is a cyclodepsipeptide, an organic disulfide and a heterocyclic antibiotic.
Romidepsin is a drug that has been approved by the U.S. Food and Drug Administration (FDA) under the brand name Istodax for the treatment of a certain type of cancer. Romidepsin is also being studied as an investigational drug as part of a strategy to cure HIV infection.As an HIV investigational drug, romidepsin belongs to a group of drugs called latency-reversing agents. Romidepsin is a selective inhibitor of histone deacetylase, approved in the US in 2009 for the treatment of cutaneous T-cell lymphoma (CTCL) in patients who have received at least one prior systemic therapy. Romidepsin is a Histone Deacetylase Inhibitor. The mechanism of action of romidepsin is as a Histone Deacetylase Inhibitor, and Bile Salt Export Pump Inhibitor, and Organic Anion Transporting Polypeptide 1B1 Inhibitor, and Organic Anion Transporting Polypeptide 1B3 Inhibitor, and Organic Anion Transporter 1 Inhibitor, and Organic Cation Transporter 2 Inhibitor. Romidepsin is an intravenously administered histone deacetylase inhibitor and antineoplastic agent that is approved for use in refractory or relapsed cutaneous and peripheral T cell lymphomas. Romidepsin is associated with modest rate of minor serum enzyme elevations during therapy but has not been linked to cases of clinically apparent liver injury, although it has been reported to cause reactivation of hepatitis B. Istodax has been reported in Humicola fuscoatra and Chromobacterium violaceum with data available. Romidepsin is a bicyclic depsipeptide antibiotic isolated from the bacterium Chromobacterium violaceum with antineoplastic activity. After intracellular activation, romidepsin binds to and inhibits histone deacetylase (HDAC), resulting in alterations in gene expression and the induction of cell differentiation, cell cycle arrest, and apoptosis. This agent also inhibits hypoxia-induced angiogenesis and depletes several heat shock protein 90 (Hsp90)-dependent oncoproteins. Drug Indication Romidepsin is indicated for the treatment of cutaneous T-cell lymphoma (CTCL) in adult patients who have received at least one prior systemic therapy. FDA Label treatment of peripheral T-cell lymphoma (PTCL), Treatment of peripheral T-cell lymphoma (nodal, other extranodal and leukaemic/disseminated) Mechanism of Action Romidepsin is a prodrug, where it becomes active once taken up into the cell. The active metabolite has a free thiol group, which interacts with zinc ions in the active site of class 1 and 2 HDAC enzymes, resulting in inhibition of its enzymatic activity. Certain tumors have over expressed HDACs and downregulated/mutated histone acetyltransferases. This imbalance of HDAC relative to histone acetyltransferase can lead to a decrease in regulatory genes, ensuing tumorigenesis. Inhibition of HDAC may restore normal gene expression in cancer cells and result in cell cycle arrest and apoptosis. |
| 分子式 |
C24H36N4O6S2
|
|
|---|---|---|
| 分子量 |
540.7
|
|
| 精确质量 |
540.207
|
|
| 元素分析 |
C, 53.31; H, 6.71; N, 10.36; O, 17.75; S, 11.86
|
|
| CAS号 |
128517-07-7
|
|
| 相关CAS号 |
|
|
| PubChem CID |
5352062
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
942.8±65.0 °C at 760 mmHg
|
|
| 熔点 |
219-224°C
|
|
| 闪点 |
524.0±34.3 °C
|
|
| 蒸汽压 |
0.0±0.3 mmHg at 25°C
|
|
| 折射率 |
1.529
|
|
| LogP |
0.95
|
|
| tPSA |
193.3
|
|
| 氢键供体(HBD)数目 |
4
|
|
| 氢键受体(HBA)数目 |
8
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
36
|
|
| 分子复杂度/Complexity |
905
|
|
| 定义原子立体中心数目 |
4
|
|
| SMILES |
S1C([H])([H])[C@]2([H])C(N([H])/C(=C(/[H])\C([H])([H])[H])/C(N([H])C([H])(C(=O)O[C@]([H])(C([H])=C([H])C([H])([H])C([H])([H])S1)C([H])([H])C(N([H])[C@@]([H])(C(N2[H])=O)C([H])(C([H])([H])[H])C([H])([H])[H])=O)C([H])(C([H])([H])[H])C([H])([H])[H])=O)=O |c:27|
|
|
| InChi Key |
OHRURASPPZQGQM-GCCNXGTGSA-N
|
|
| InChi Code |
InChI=1S/C24H36N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15-9-7-8-10-35-36-12-17(22(31)25-16)26-23(32)19(13(2)3)27-18(29)11-15/h6-7,9,13-15,17,19-20H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17-,19-,20+/m1/s1
|
|
| 化学名 |
(1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-di(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetrazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone
|
|
| 别名 |
NSC 630176;FK228; FK 228; FK-228; FR901228; FR-901228; FR 901228; NSC-630176; NSC-630176; depsipeptide; US trade name: Istodax.
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 该产品在溶液状态不稳定,请现配现用。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (3.85 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.08 mg/mL (3.85 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (3.85 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 1% DMSO+30% polyethylene glycol+1% Tween 80: 18mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8495 mL | 9.2473 mL | 18.4945 mL | |
| 5 mM | 0.3699 mL | 1.8495 mL | 3.6989 mL | |
| 10 mM | 0.1849 mL | 0.9247 mL | 1.8495 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03547700 | Active Recruiting |
Drug: Romidepsin Drug: Ixazomib |
Lymphoma, T-Cell, Peripheral | Ryan Wilcox | November 18, 2021 | Phase 1 Phase 2 |
| NCT01638533 | Active Recruiting |
Other: Pharmacological Study Drug: Romidepsin |
Glioma Lymphoma |
National Cancer Institute (NCI) |
June 12, 2012 | Phase 1 |
| NCT02393794 | Active Recruiting |
Drug: Romidepsin Drug: Cisplatin Drug: Nivolumab |
Breast Cancer Triple-Negative Breast Cancer |
Priyanka Sharma | July 17, 2015 | Phase 1 Phase 2 |
| NCT02616965 | Active Recruiting |
Drug: Brentuximab vedotin Drug: Romidepsin |
Cutaneous T-cell Lymphoma (CTCL) |
Fox Chase Cancer Center | February 22, 2017 | Phase 1 |
| NCT04747236 | Recruiting | Drug: Romidepsin Drug: Azacytidine |
PTCL | University of Virginia | February 19, 2021 | Phase 2 |
|
|
|
|
|
|
|