| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
PDE4 ( IC50 = 3-240 nM)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
免疫纯化的 PDE4B 和 PDE4D 的活性也受到 PDE4 选择性抑制剂 Rolipram 的抑制,IC50 值分别为 130 nM 和 240 nM。相反,免疫纯化的 PDE4A 活性被 Rolipram 抑制,尽管 IC50 低得多,约为 3 nM。咯利普兰以剂量依赖性方式增加 U937 细胞中 cAMP 反应元件结合蛋白 (CREB) 磷酸化,表明存在高亲和力和低亲和力成分(分别为 IC50 ~1 nM 和 IC50 ~120 nM)。当 IFN-γ 刺激时,咯利普兰的 IC50 为 290 nM,剂量依赖性且简单且单调地抑制 p38 MAPK 的磷酸化 [1]。所有四种 PDE4 异构体均被选择性 PDE4 抑制剂咯利普兰抑制。 Rolipram 以最大/次最大方式和剂量依赖性方式抑制 LPS 诱导的 TNF 产生(IC50 为 25.9 nM)。在 2 μM 的剂量下,在 J774 细胞中发现了抑制作用 [2]。
|
||
| 体内研究 (In Vivo) |
咯利普兰似乎降低了 LPS 在 WT 小鼠腹腔巨噬细胞 (PM) 中产生的 TNF mRNA 和蛋白表达(TNF mRNA 和 TNF 蛋白分别抑制 74% 和 63%)。与之前的发现一致,MKP-1 (-/-) 小鼠的 PM 中 LPS 诱导的 TNF 生成量高于 WT 小鼠的 PM。有趣的是,Rolipram 对 TNF mRNA 和蛋白表达的抑制作用大大降低,并且在 MKP-1 (-/-) 小鼠的 PM 中并未达到统计学显着性 [2]。在训练有素的无助大鼠中,重复腹腔注射咯利普兰(1.25 mg/kg)可以减少未成功逃脱尝试的次数[3]。
|
||
| 酶活实验 |
免疫沉淀和PDE测定[1]
如前所述,对四种PDE4类酶进行选择性免疫沉淀。如前所述,使用足够的抗血清来确保靶PDE4亚类的所有同工酶被选择性免疫沉淀;然后对这些进行PDE测定。PDE测定是通过修改两步Thompson和Appleman方法完成的。用新鲜细胞裂解物测定总细胞PDE活性和PDE3和PDE4组分的量。如前所述,使用1µM cAMP作为底物和10µM PDE3选择性抑制剂西洛司胺或PDE4选择性抑制剂rolipram/罗利普兰来测定PDE3和PDE4的总活性。 |
||
| 细胞实验 |
J774小鼠巨噬细胞在37°C、5%CO2气氛下在DMEM中培养,DMEM补充了含有10%热灭活FBS、100 U·mL-1青霉素、100μg·mL-1链霉素和250 ng·mL-1两性霉素B的谷氨酸-1。在实验中,细胞以每孔2×105个细胞的密度接种在24孔板上。在实验开始之前,细胞单层生长72小时Rolipram、IBMX和BIRB 796溶解在二甲亚砜(DMSO)中,8-Br-cAMP溶解在HBSS中。将LPS(10 ng·mL-1)或指定浓度的目标化合物或溶剂(DMSO,0.1%v/v)加入含有10%FBS和补充剂的新鲜培养基中的细胞中。将细胞进一步孵育指定时间。
通过细胞增殖试剂盒II(XTT)评估LPS和受试化学物质对细胞存活率的影响。没有观察到LPS或实验中使用的其他化学物质会引起细胞毒性。[2]
|
||
| 动物实验 |
|
||
| 毒性/毒理 (Toxicokinetics/TK) |
mouse LD oral >300 mg/kg Biological and Pharmaceutical Bulletin., 17(498), 1994 [PMID:8069256]
|
||
| 参考文献 |
|
||
| 其他信息 |
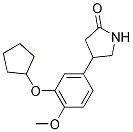
Rolipram is a member of the lclass of pyrrolidin-2-ones that is pyrrolidin-2-one bearing a 3-(cyclopentyloxy)-4-methoxyphenyl substituent at the 4-position. It is a type IV-specific phosphodiesterase (PDE4) inhibitor. It has a role as an antidepressant and an EC 3.1.4.* (phosphoric diester hydrolase) inhibitor.
A phosphodiesterase inhibitor with antidepressant properties. A phosphodiesterase 4 inhibitor with antidepressant properties. U937 monocytic cells are shown here to express a range of PDE4, cAMP-specific phosphodiesterase (PDE) isoenzymes: the long isoenzymes, PDE4A4, PDE4D5 and PDE4D3, plus the short isoenzyme, PDE4B2. These isoenzymes provide around 76% of the total cAMP PDE activity of U937 cells. The specific activities of the total PDE4A, PDE4B and PDE4D activities were 0.63+/-0.09, 8.8+/-0.2 and 34.4+/-2.9 pmol/min per mg of protein respectively. The PDE4 selective inhibitor, rolipram, inhibited immunopurified PDE4B and PDE4D activities similarly, with IC(50) values of approx. 130 nM and 240 nM respectively. In contrast, rolipram inhibited immunopurified PDE4A activity with a dramatically lower IC(50) value of around 3 nM. Rolipram increased phosphorylation of cAMP-response-element-binding protein (CREB) in U937 cells in a dose-dependent fashion, which implied the presence of both high affinity (IC(50) value approx. 1 nM) and low affinity (IC(50) value approx. 120 nM) components. Rolipram dose-dependently inhibited the interferon-gamma (IFN-gamma)-stimulated phosphorylation of p38 mitogen-activated protein (MAP) kinase in a simple monotonic fashion with an IC(50) value of approx. 290 nM. On this basis, it is suggested that rolipram inhibition of PDE4A4 is involved in regulating CREB phosphorylation but not IFN-gamma-stimulated p38 MAP kinase phosphorylation. PDE4A4 was also selectively activated by challenge of U937 cells with either bacterial lipopolysaccharide (LPS) or IFN-gamma through a process which was attenuated by both wortmannin and rapamycin. It is proposed that the PDE4A4 isoform is involved in compartmentalized cAMP signalling responses in U937 monocytes.[1] Background and purpose: 3',5'-Cyclic nucleotide PDE4 is expressed in several inflammatory and immune cells, and PDE4 catalyses the hydrolysis of cAMP to 5'AMP, down-regulating cAMP signalling in cells. MAPK phosphatase-1 (MKP-1) is an endogenous p38 MAPK signalling suppressor and limits inflammatory gene expression and inflammation. In the present study, we investigated the effect of a PDE4 inhibitor rolipram on MKP-1 expression and whether MKP-1 is involved in the anti-inflammatory effects of rolipram. Experimental approach: The effect of rolipram on TNF production was investigated in J774 mouse macrophage cell line and in primary mouse peritoneal macrophages (PM) from wild-type (WT) and MKP-1(-/-) mice. We also investigated the effect of rolipram on carrageenan-induced paw inflammation in WT and MKP-1(-/-) mice. Key results: MKP-1 expression was enhanced by rolipram, by a non-selective PDE inhibitor IBMX and by a cAMP analogue 8-Br-cAMP in J774 cells and in PM. Enhanced MKP-1 mRNA expression by rolipram was reversed by a PKA inhibitor. Rolipram, IBMX and 8-Br-cAMP also inhibited TNF production in activated macrophages. Accordingly, rolipram inhibited TNF production in PMs from WT mice but, interestingly, not in PMs from MKP-1(-/-) mice. Furthermore, rolipram attenuated carrageenan-induced paw inflammation in WT but not in MKP-1(-/-) mice. Conclusions and implications: PDE4 inhibitor rolipram was found to enhance the expression of MKP-1, and MKP-1 mediated, at least partly, the anti-inflammatory effects of PDE4 inhibition. The results suggest that compounds that enhance MKP-1 expression and/or MKP-1 activity hold potential as novel anti-inflammatory drugs.[2] Objectives: To investigate the alterations in GABA levels by rolipram in the model of depression. Materials and methods: The alteration of GABA content by rolipram as a phosphodiesterase enzyme type-4 inhibitor in the frontal cortex (FCx; as a brain region crucial for the control of emotion and cognition) obtained from male mice exposed to chronic mild stress (CMS)-induced anhedonia (the loss of pleasure or lack of sensitivity to pleasure stimuli) was recorded. Results: The results demonstrated the reversal of CMS-induced anhedonia after 3 weeks per os of rolipram in a dose of 0.1 mg/kg/day dissolved in distilled water. Furthermore, rolipram showed a significant reduction in duration of immobility in long-term behavioral changes recorded by the FST. Additionally, there was a significant increase in the GABA content of the FCx of rolipram-treated mice exposed to CMS-induced anhedonia. Conclusions: The present study suggested that GABA levels may be decreased in an animal model of depression and its reversal together with the behaviour improvement by rolipram could support the hypothesis that modification in GABAergic activity has a role in mood disorders. These effects may complement the antidepressant effect of rolipram that is originally mediated via inhibition of phosphodiesterase enzyme type-4 [PDE4] that increases cyclic adenosine monophosphate signalling the pharmacotherapy of depression.[3] |
| 分子式 |
C16H21NO3
|
|---|---|
| 分子量 |
275.34
|
| 精确质量 |
275.152
|
| 元素分析 |
C, 69.79; H, 7.69; N, 5.09; O, 17.43
|
| CAS号 |
61413-54-5
|
| 相关CAS号 |
(R)-(-)-Rolipram;85416-75-7;(S)-(+)-Rolipram;85416-73-5
|
| PubChem CID |
5092
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
472.7±45.0 °C at 760 mmHg
|
| 熔点 |
127-133ºC
|
| 闪点 |
239.7±28.7 °C
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
| 折射率 |
1.552
|
| LogP |
1.43
|
| tPSA |
47.56
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
20
|
| 分子复杂度/Complexity |
341
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
HJORMJIFDVBMOB-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18)
|
| 化学名 |
4-(3-(cyclopentyloxy)-4-methoxyphenyl)pyrrolidin-2-one
|
| 别名 |
ZK-62711; SB 95952; SB95952; rolipram; 61413-54-5; (+/-)-Rolipram; (R,S)-rolipram; ZK 62711; Rolipramum [Latin]; 4-(3-(cyclopentyloxy)-4-methoxyphenyl)pyrrolidin-2-one; Rolipramum; SB-95952; ME-3167; ZK-62711; ME3167; ZK62711; ME 3167; ZK 62711
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (9.08 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (9.08 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (9.08 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 30% PEG400+0.5% Tween80+5% propylene glycol:10 mg/L 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.6319 mL | 18.1594 mL | 36.3187 mL | |
| 5 mM | 0.7264 mL | 3.6319 mL | 7.2637 mL | |
| 10 mM | 0.3632 mL | 1.8159 mL | 3.6319 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05522673 | Terminated Has Results | Drug: 11(R)-rolipram | Depression | National Institute of Mental Health (NIMH) |
February 8, 2023 | Phase 1 |
| NCT00011375 | Completed | Drug: Rolipram | Multiple Sclerosis | National Institute of Neurological Disorders and Stroke (NINDS) |
February 2001 | Phase 2 |
| NCT01215552 | Terminated | Drug: HT-0712 | Healthy Elderly Volunteers | Dart NeuroScience, LLC | September 2010 | Phase 1 |
| NCT00250172 | Completed | Drug: [C-11](R)-rolipram | Dosimetry Healthy |
National Institute of Mental Health (NIMH) |
October 31, 2005 | Phase 1 |
|
|---|
|
|