| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
Kir3.2 转化细胞的生长和 Kir3.2 活性受到原黄素(0.1-10 μM;24 小时)的浓度依赖性抑制[1]。丙黄素 (300 μM) 使 Kir3.2 突变体的电流幅度逐渐降低至对照的 27.7±4.3% [2]。
|
|---|---|
| 体内研究 (In Vivo) |
静脉注射后全血中的丙黄素(20 mg/kg)浓度迅速下降,约半小时后稳定[3]。
|
| 细胞实验 |
细胞活力测定 [2]
细胞类型: Kir3.2* 转化体 BYT123 细胞 测试浓度: 0.1、1 和 10 μM 孵育持续时间:24 小时 实验结果:Kir3.2* 转化细胞生长的剂量依赖性抑制。减弱 Kir3.2* 转化细胞的生长,而不影响对照细胞的生长。 |
| 动物实验 |
Animal/Disease Models: Adult male Sprague Dawley rats (body weight approximately 200 g) [3]
Doses: 20 mg/kg (pharmacokinetic/PK/PK analysis) Route of Administration: intravenous (iv) (iv)injection; 20 mg/kg. Results at 2, 4, 5, 10, 15, 20, 25 and 30 minutes after Route of Administration: Whole blood concentration diminished rapidly in the first 5 minutes after administration, and then diminished slowly. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The uptake of the fluorescent drug proflavine was measured in suspensions of hepatocytes from normal and carcinogen (2-acetylaminofluorine, AAF)-fed rats by flow cytometry. Drug uptake into hepatocytes from carcinogen-fed animals was consistently lower than that into hepatocytes from normal animals. Isolated nuclei, prepared from the livers of normal and AAF-fed rats showed similar proflavine uptake. Drug uptake into hepatocytes from AAF-fed animals, however, was increased by prior exposure to a metabolic inhibitor. Thus, differences in drug uptake may reflect changes in the cell membrane, together with an alteration in the metabolic integrity of the cells. The uptake of drug in hepatocytes from AAF-fed rats was uniformly low within each cell preparation. However, drug uptake varied not only between tumours arising in the livers of these animals but also within each tumour cell preparation. This study indicates that flow cytometry can provide an effective means for analysing drug uptake into cell populations arising during hepatocarcinogenesis. 1. The disposition of proflavine (PRO) and acriflavine (ACR) were examined in channel catfish after intravascular (i.v.) dosing (1 mg/kg) or waterborne exposure (10 mg/l for 4 h). 2. After i.v. dosing, plasma concentration-time profiles of parent PRO and ACR were best described by two- and three-compartment pharmacokinetic models respectively. Terminal elimination half-lives of PRO and ACR in plasma were 8.7 and 11.4 h respectively. 3. In animals dosed with 14C-PRO or 14C-ACR, total drug equivalent concentrations were highest in the excretory organs and lowest in muscle, fat and plasma. In PRO-dosed animals, residues in the liver and trunk kidney were composed primarily of glucuronosyl and acetyl conjugates of PRO; residues in muscle were composed mostly (> 95%) of the parent drug. In ACR-dosed animals, the parent compound comprised > 90% of the total residues in all tissues examined. 4. PRO and ACR were poorly absorbed in catfish during waterborne exposure. At the end of a 4-h exposure, parent PRO and ACR concentrations in muscle were 0.064 and 0.020 microgram/g respectively. Levels in muscle declined below the limit of determination (0.005 microgram/g) within 1-2 weeks. Metabolism / Metabolites Proflavine (3,6-diaminoacridine) has potential for use as an antiinfective in fish, and its metabolism by rainbow trout was therefore studied. Fourteen hours after intraarterial bolus administration of 10 mg/kg of proflavine, three metabolites were found in liver and bile, and one metabolite was found in plasma using reversed-phase HPLC with UV detection at 262 nm. Treatment with hydrochloric acid converted the three metabolites to proflavine, which suggested that the metabolites were proflavine conjugates. Treatment with beta-glucuronidase and saccharic acid 1,4-lactone, a specific beta-glucuronidase inhibitor, revealed that two metabolites were proflavine glucuronides. For determination of UV-VIS absorption and mass spectra, HPLC-purified metabolites were isolated from liver. Data from these experiments suggested that the proflavine metabolites were 3-N-glucuronosyl proflavine (PG), 3-N-glucuronosyl,6-N-acetyl proflavine (APG), and 3-N-acetylproflavine (AP). The identities of the metabolites were verified by chemical synthesis. When synthetic PG and AP were compared with the two metabolites isolated from trout, they had the same molecular weight as determined by matrix-assisted, laser desorption ionization, time-of-flight MS. In addition, they coeluted on HPLC under different mobile phase conditions. Finally, the in vitro incubation with liver subcellular preparations confirmed this characterization and provided the evidence that APG can be formed by glucuronidation of AP or acetylation of PG. A liquid chromatographic (LC) method was developed for determination of acriflavine (ACR) and proflavine (PRO) residues in channel catfish muscle. Residues were extracted with acidified methanol solution, and extracts were cleaned up with C18 solid-phase extraction columns. Residue concentrations were determined on an LC cyano column, with spectrophotometric detection at 454 nm. Catfish muscle was individually fortified with ACR (purified from commercial product) and PRO at concentrations of 5, 10, 20, 40, and 80 ppb (5 replicates per level). Mean recoveries from fortified muscle at each level ranged from 86 to 95%, with relative standard deviations (RSDs) of 2.5 to 5.7%. The method was applied to incurred residues of ACR and PRO in muscle after waterborne exposure of channel catfish to commercial acriflavine (10 ppm total dye for 4 h). RSDs for incurred residues of ACR and PRO were in the same range as those for fortified muscle. Low residue concentrations (< 1% of exposure water concentration) suggested poor absorption of ACR and PRO in catfish. |
| 毒性/毒理 (Toxicokinetics/TK) |
Non-Human Toxicity Values
LD50 mouse intraperitoneal 50 mg/kg LD50 mouse subcutaneous 140 mg/kg |
| 参考文献 |
|
| 其他信息 |
3,6-diaminoacridine is an aminoacridine that is acridine that is substituted by amino groups at positions 3 and 6. A slow-acting bacteriostat that is effective against many Gram-positive bacteria (but ineffective against spores), its salts were formerly used for treatment of burns and infected wounds. It has a role as an antiseptic drug, a carcinogenic agent, an antibacterial agent, a chromophore and an intercalator. It is a conjugate base of a 3,6-diaminoacridine(1+).
3,6-Diaminoacridine. Topical antiseptic used mainly in wound dressings. Proflavine Hemisulfate is the hemisulfate salt form of proflavine, an acridine-derived fluorescent contrast and disinfectant agent that can potentially be used for cellular imaging and antiseptic purposes. Upon topical application of proflavine hemisulfate, proflavine diffuses into cells and intercalates into DNA, thereby accumulating in and staining the nucleus. During fluorescence imaging, the cell nuclei can be visualized. This allows nuclear morphometry and the identification of cancer cells. In addition, proflavine exerts its antibacterial effect by binding to bacterial DNA, thereby disrupting DNA synthesis and halting bacterial cell growth. Topical antiseptic used mainly in wound dressings. Drug Indication Topical antiseptic used mainly in wound dressings. Mechanism of Action Proflavine acts by interchelating DNA (intercalation), thereby disrupting DNA synthesis and leading to high levels of mutation in the copied DNA strands. This prevents bacterial reproduction. The ability of proflavine (3,6-diaminoacridine) and its 2,7-dimethyl, 2,7-diethyl, 2,7-diisopropyl and 2,7-di-tert.-butyl derivatives to induce the 'petite' mutation in Saccharomyces cerevisiae has been studied in relation to the DNA-binding properties of the compounds. The nature of the binding has been investigated by nuclear magnetic resonance techniques, and the results support and clarify earlier suggestions that the first 3 members of the series intercalate into DNA while the diisopropyl and di-tert.-butyl compounds do not. Toxicity of the drugs was primarily associated with their mode of DNA binding, but lipophilicity had an important secondary effect. It seems likely that the toxic properties of the more lipophilic DNA-intercalating members of the series mask their potential for 'petite' mutagenesis. The toxicities of several aminoacridines were measured against pathogenic strains of both Gram-positive (Staphylococcus aureus, Enterococcus faecalis, Bacillus cereus) and Gram-negative (Escherichia coli, Pseudomonas aeruginosa) organisms. In several cases, illumination at a light dose of 6.3 J/cm2 resulted in considerable decreases in the minimum lethal drug concentrations required, giving up to 50-fold increases in bactericidal activity. Derivatives of 9-aminoacridine (aminacrine) exhibited phototoxicity against one or more of the test organisms, but the established photosensitizing acridines proflavine and acridine orange were photobactericidal against all strains. Therapeutic Uses /Exptl/ We used the photodynamic inactivation technique with proflavine as the photoactive dye to treat herpetic epithelial keratitis in a preliminary study of patients who had idoxuridine toxicity or resistance. A comparative study with idoxuridine in treating dendritic ulcerations of the cornea showed a good therapeutic effect. But the investigation was suspended when adverse reactions, consisting of a generalized epithelial keratitis and an anterior uveitis, possibly of phototoxic origin, developed in a few patients receiving treatment. The ulcers treated by photodynamic inactivation apparently healed by a process of "debridement" followed by subsequent re-epithelialization. ... Proflavine wool is used by many surgeons in the UK as a dressing that can be moulded to conform to the contours of a corrected prominent ear. ... Drug Warnings Proflavine allergy is uncommon, occurring in approximately 6% of patients attending contact dermatitis clinics. Proflavine wool is used by many surgeons in the UK as a dressing that can be moulded to conform to the contours of a corrected prominent ear. It may have bacteriostatic properties. We present a case where contact dermatitis in response to proflavine developed after pinnaplasty. This caused diagnostic confusion, a lengthened hospital stay and an unsightly hypertrophic scar. Pharmacodynamics Proflavine is an acriflavine derivative which is a disinfectant bacteriostatic against many gram-positive bacteria. Proflavine is toxic and carcinogenic in mammals and so it is used only as a surface disinfectant or for treating superficial wounds. |
| 精确质量 |
209.095
|
|---|---|
| CAS号 |
1811-28-5
|
| 相关CAS号 |
Proflavine;92-62-6;Proflavine dihydrochloride;531-73-7
|
| PubChem CID |
7099
|
| 外观&性状 |
Brown to reddish brown solid powder
|
| 密度 |
1.346 g/cm3
|
| 沸点 |
506.9ºC at 760 mmHg
|
| 熔点 |
284-286ºC
|
| 闪点 |
292.9ºC
|
| 蒸汽压 |
0mmHg at 25°C
|
| LogP |
3.714
|
| tPSA |
64.93
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
0
|
| 重原子数目 |
16
|
| 分子复杂度/Complexity |
232
|
| 定义原子立体中心数目 |
0
|
| SMILES |
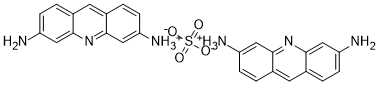
NC1=CC2=NC3=CC(N)=CC=C3C=C2C=C1.[0.5H2SO4]
|
| InChi Key |
WDVSHHCDHLJJJR-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C13H11N3/c14-10-3-1-8-5-9-2-4-11(15)7-13(9)16-12(8)6-10/h1-7H,14-15H2
|
| 化学名 |
acridine-3,6-diamine
|
| 别名 |
Proflavine hemisulfate dihydrate 3,6 Diamino Acridine 3,6 Diaminoacridine 3,6-diamino acridine Proflavine hemisulfate EINECS 217-320-3 3,6-Acridinediamine, sulfate (2
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ≥ 5 mg/mL (~19.36 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01384227 | COMPLETED | Drug: Proflavine Hemisulfate | Barrett's Esophagus | Anandasabapathy, Sharmila, M.D | 2009-02 | Early Phase 1 |
| NCT01384240 | TERMINATED | Drug: Proflavine Hemisulfate | Anal Dysplasia Colon Polyps Colonic Dysplasia |
Anandasabapathy, Sharmila, M.D | 2010-04 | Early Phase 1 |
| NCT01384695 | TERMINATED | Drug: Fluorescein Drug: Proflavine hemisulfate |
Barrett's Esophagus GERD |
Anandasabapathy, Sharmila, M.D | 2009-06 | Early Phase 1 |
| NCT01384708 | COMPLETED | Drug: proflavine | Squamous Cell Cancer | Anandasabapathy, Sharmila, M.D | 2010-08 | Early Phase 1 |
| NCT01384864 | COMPLETED | Drug: proflavine | Barrett's Esophagus Intraepithelial Neoplasia |
Anandasabapathy, Sharmila, M.D | 2011-08 | Early Phase 1 |