| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Pyrethrins are absorbed from the gastrointestinal tract following oral administration. Studies in male rats receiving 3 mg/kg orally resulted in almost complete absorption and metabolism within 100 hours. No pyrethrin was observed in urine, although substantial quantities of metabolites were present. In feces, small quantities of the parent pyrethrin were observed, again accompanied by metabolites. Pyrethrins are absorbed through intact skin when applied topically. When animals were exposed to aerosols of pyrethrins with piperonyl butoxide being released into the air, little or none of the combination was systemically absorbed. /Pyrethrins/ The pyrethrins or their metabolites are not known to be stored in the body or to be excreted in the milk... Following a single oral pyrethrin II dose to rats, 53% of the admin dose appeared as CO2 & 7% appeared in urine. After an equivalent dose of pyrethrin I, 0.3% could be accounted for as CO2 & 46% of the dose was eliminated in urine. Metabolism / Metabolites Pyrethrins are extensively metabolized, the residues of the parent compound in feces and urine representing only 10%. Six metabolites were identified and two major metabolic pathways were suggested, the first involving oxidation of the double-bond and/or the methyl groups and the second involving hydrolysis of the ester bond. Pyrethrins I are metabolized mainly through oxidative processes, while pyrethrins II are metabolized through a combination of hydrolytic and oxidative processes. ... Within 48 hr of oral admin of (14)C-pyrethrin II to rats, 53% of the (14)C was recovered as exhaled carbon dioxide ... . The ... (14)C recovered from urine ... /was/ 7% ... some of the orally admin material is excreted in feces, at least partially in metabolized form. Three compounds have been isolated from urine & identified by NMR & mass spectra. All three are produced by ... pyrethrin I & II. All three are the result of oxidation of ... the acid & alcoholic moieties leaving the main structure of the molecule intact. The oral administration of radio-labelled pyrethrin I, or pyrethrin II, to rats produced several urinary metabolites. Each contained a trans-2-carboxyprop-1-enyl side chain resulting from oxidation of the chrysanthemate isobutenyl group or hydrolysis of the pyrethrate methoxy-carbonyl group. Also, the cis-2',4'-pentadienyl side chain of pyrethrin I and pyrethrin II was oxidized at the penta-2,4-dienyl group to give a cis-4',5'-dihydroxypent-2'-enyl group, a 4' conjugate of this diol, or a trans-2',5'-dihydroxypent-3'-enyl group. The 2-methylpropenyl group of (S)-bioallethrin (A) and the pentadienyl group of pyrethrin II are selectively oxidized by m-chloroperoxybenzoic acid in dichloromethane to yield the 7,8-epoxide (1) from A and a mixture of the 8',9'- and 10',11'-epoxides (7 and 8) from pyrethrin II. These epoxides are hydrated in aqueous acid to the corresponding diols and other hydroxy derivatives produced by opening of the cycloprophyl ring or migration of the adjacent double bond. The epoxy and hydroxy derivatives are identified by two dimensional NMR techniques. Mouse liver enzymes do not detectably hydrate epoxide 1 but quickly hydrate epoxides 7 and 8 without migration of the double bond. HPLC analyses of the microsomal metabolites of pyrethrins I and II identify the 10',11'-diols as major metabolites and the 8',9'-diols as minor products. For more Metabolism/Metabolites (Complete) data for PYRETHRIN II (12 total), please visit the HSDB record page. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
Piperonyl butoxide potentiates /insecticidal activity/ of pyrethrins by inhibiting the hydrolytic enzymes responsible for pyrethrins' metabolism in arthropods. When piperonyl butoxide is combined with pyrethrins, the insecticidal activity of the latter drug is increased 2-12 times /Pyrethrins/ At dietary level of 1000 ppm pyrethrins & 10000 ppm piperonyl butoxide ... /enlargement, margination, & cytoplasmic inclusions in liver cells of rats/ were well developed in only 8 days, but ... were not maximal. Changes were proportional to dosage & similar to those produced by DDT. Effects of the 2 ... were additive. /Pyrethrins/ There is no evidence that synergists incr toxicity of the pyrethrins to mammals. /pyrethrins/ Antioxidants used to help protect insecticidal residues of pyrethrins include minute concn of pyrocatechol, pyrogallol, & hydroquinone; 1-benzene-azo-2-naphthol is used to protect against the effects of sunlight. /LABORATORY ANIMALS: Developmental or Reproductive Toxicity/ ... Extract containing pyrethrum & piperonyl butoxide /was applied/ to chorio-allantoic membrane /of chick embryo/ & produced damaged testes with absence of gonadocytes in the surviving chicken embryo. /Pyrethrum extract/ Non-Human Toxicity Values LD50 Rat male oral greater than 600 mg/kg LD50 Mouse intraperitoneal less than 240 mg/kg LD50 Cat female intravenous 1 mg/kg LD50 Rat oral 1.2 g/kg For more Non-Human Toxicity Values (Complete) data for PYRETHRIN II (8 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
Pyrethrin II is a member of pyrethrins. It is functionally related to a pyrethrin I.
Mechanism of Action The symptoms of pyrethrin poisoning follow the typical pattern ... : (1) excitation, (2) convulsions, (3) paralysis, and (4) death. The effects of pyrethrins on the insect nervous system closely resemble those of DDT, but are apparently much less persistent. Regular, rhythmic, and spontaneous nerve discharges have been observed in insect and crustacean nerve-muscle preparations poisoned with pyrethrins. The primary target of pyrethrins seems to be the ganglia of the insect central nervous system although some pyrethrin-poisoning effect can be observed in isolated legs. /Pyrethrins/ Electrophysiologically, pyrethrins cause repetitive discharges and conduction block. /Pyrethrins/ The primary site of action for pyrethrins ... is the sodium channel of nerve cells. Using a variety of methods, including voltage clamp and patch clamp techniques, it has been shown that pyrethrins ... slow the closing of sodium channel gates following an initial influx of sodium during the depolarizing phase of an action potential, which results in a prolonged sodium tail current. Following absorption through the chitinous exoskeleton of arthropods, pyrethrins stimulate the nervous system, apparently by competitively interfering with cationic conductances in the lipid layer of nerve cells, thereby blocking nerve impulse transmissions. Paralysis & death follow. /Pyrethrins/ The interactions of natural pyrethrins and 9 pyrethroids with the nicotinic acetylcholine (ACh) receptor/channel complex of Torpedo electronic organ membranes were studied. None reduced (3)H-ACh binding to the receptor sites, but all inhibited (3)H-labeled perhydrohistrionicotoxin binding to the channel sites in presence of carbamylcholine. Allethrin inhibited binding noncompetitively, but (3)H-labeled imipramine binding competitively, suggesting that allethrin binds to the receptor's channel sites that bind imipramine. The pyrethroids were divided into 2 types according to their action: type A, which included allethrin, was more potent in inhibiting (3)H-H12-HTX binding and acted more rapidly. Type B, which included permethrin, was less potent and their potency increased slowly with time. The high affinities that several pyrethroids have for this nicotinic ACh receptor suggest that pyrethroids may have a synaptic site of action in addition to their well known effects on the axonal channels. /Pyrethrins and Pyrethroids/ Therapeutic Uses ... The insecticide has been considered so innocuous that an ointment containing 0.75% pyrethrin was recommended for treatment of scabies, and such use led to only a few cases of dermatitis, some of doubtful relation to the treatment. Pyrethrins have been used extensively for the control of human body lice. Head lice can be treated with pyrethrin 0.3% plus 3% piperonyl butoxide. Drug Warnings Because commercial formulations are irritating to the eyes and mucous membranes, they should not be used to treat P. pubis infestations of the eyelashes. Individuals sensitive to ragweed have shown cross-sensitivity to unrefined but not to refined pyrethrins; however, manufacturers of pyrethrins combinations warned that these products should not be used by ragweed-sensitive patients. /Pyrethrins/ Local irritation incl erythema, pruritus, urticaria, edema, eczema, & slight corneal erosion & stromal edema may occur & ... contact with face, eyes, mucous membranes, & urethral meatus should be avoided. ... Pyrethrins with piperonyl butoxide should not be applied to acutely inflamed skin or raw, weeping surfaces. ... The drug should not be used more than twice in 24 hours. /Pyrethrins/ |
| 分子式 |
C22H28O5
|
|---|---|
| 分子量 |
372.45
|
| 精确质量 |
372.193
|
| CAS号 |
121-29-9
|
| PubChem CID |
5281555
|
| 外观&性状 |
Light yellow to yellow liquid
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
473.7±45.0 °C at 760 mmHg
|
| 闪点 |
203.8±28.8 °C
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
| 折射率 |
1.528
|
| LogP |
4.43
|
| tPSA |
69.67
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
751
|
| 定义原子立体中心数目 |
3
|
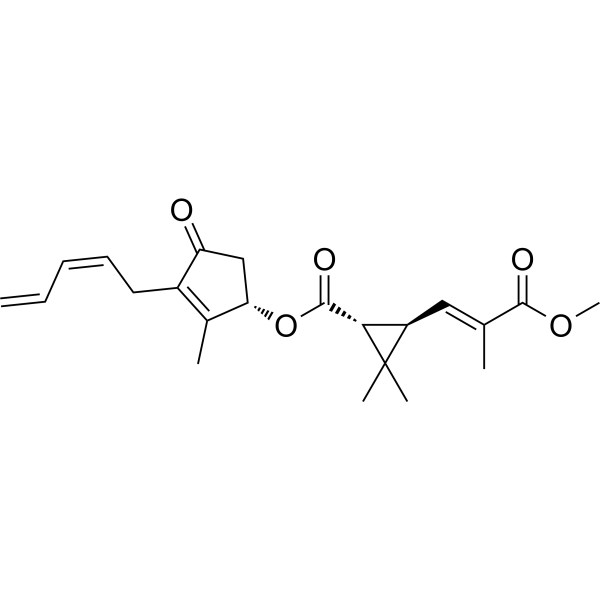
| SMILES |
CC1=C(C(=O)C[C@@H]1OC(=O)[C@@H]2[C@H](C2(C)C)/C=C(\C)/C(=O)OC)C/C=C\C=C
|
| InChi Key |
VJFUPGQZSXIULQ-XIGJTORUSA-N
|
| InChi Code |
InChI=1S/C22H28O5/c1-7-8-9-10-15-14(3)18(12-17(15)23)27-21(25)19-16(22(19,4)5)11-13(2)20(24)26-6/h7-9,11,16,18-19H,1,10,12H2,2-6H3/b9-8-,13-11+/t16-,18+,19+/m1/s1
|
| 化学名 |
[(1S)-2-methyl-4-oxo-3-[(2Z)-penta-2,4-dienyl]cyclopent-2-en-1-yl] (1R,3R)-3-[(E)-3-methoxy-2-methyl-3-oxoprop-1-enyl]-2,2-dimethylcyclopropane-1-carboxylate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO :~100 mg/mL (~268.49 mM; with sonication)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.71 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween-80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加),澄清溶液。
例如,若需制备1 mL的工作液,您可以添加 100 μL 25.0 mg/mL 透明 DMSO 储备液,并将其添加到 400 μL PEG300 中并充分混合。 然后向上述体系中加入50 μL Tween-80,混匀。 然后继续加入450 μL生理盐水至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6849 mL | 13.4246 mL | 26.8492 mL | |
| 5 mM | 0.5370 mL | 2.6849 mL | 5.3698 mL | |
| 10 mM | 0.2685 mL | 1.3425 mL | 2.6849 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。