| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
| 靶点 |
Neuropeptide Y (NPY) Y1 receptor
|
|---|---|
| 体外研究 (In Vitro) |
1.合成了新型Y1选择性精氨酸衍生物BIBO 3304((R)-N-[[4-(氨基羰基氨基甲基)-苯基]甲基]-N2-(二苯乙酰基)-精氨酸三氟乙酸酯),并对其亚型选择性、体外拮抗性和食物摄入抑制性进行了检测。2.BIBO 3304对人和大鼠Y1受体均显示亚纳米亲和力(IC50值分别为0.38+/-0.06 nM和0.72+/-0.42 nM)。BIBO 3304(BIBO 3457)的非活性对映体对人和大鼠Y1受体亚型的亲和力都很低(IC50>1000 nM)。BIBO 3304对人Y2受体、人和大鼠Y4受体以及人和大白鼠Y5受体显示出低亲和力(IC50值>1000 nM)。[1]
为了测试Y1受体信号在人胰岛中的生理相关性,在Y1受体拮抗剂BIBO3304存在或不存在的情况下,用葡萄糖培养和刺激人胰岛。与啮齿动物的胰岛类似,用BIBO3304处理的人胰岛在葡萄糖挑战下表现出增强的胰岛素分泌[2]。 如图4f所示,与单独使用葡萄糖相比,在100 nM艾塞那肽和20 mM葡萄糖的存在下,cAMP显著上调。添加50 nM PYY能够显著减少艾塞那肽诱导的cAMP增加,并且在Y1受体特异性拮抗剂BIBO3304[2]的存在下,这种抑制作用被消除。 |
| 体内研究 (In Vivo) |
BIBO3304 TFA(30 μg;双侧室旁核注射)可减少空腹后食欲亢进[1]。 BIBO3304 TFA (15-60 μg) 剂量依赖性地抑制 1 μg NPY 介导的进食反应 [1]。 BIBO3304 TFA(0.5 μM;口服)可显着提高血液胰岛素水平 [2]。
3.脑室旁核注射30微克BIBO 3304抑制了1微克NPY诱导的摄食反应以及24小时禁食诱导的摄食过度,这暗示Y1受体在NPY介导的摄食中起作用。非活性对映体没有影响。4.BIBO 3304既不抑制甘丙肽,也不抑制去甲肾上腺素诱导的食欲反应。但它阻断了[Leu31、Pro24]NPY和NPY(3 36)引起的摄食行为,表明不同NPY受体亚型在摄食行为中相互作用。5.本研究表明,BIBO 3304是一种亚型选择性非肽拮抗剂,对Y1受体亚型具有亚纳米亲和力,可显著抑制NPY或禁食诱导的食物摄入[1]。 接受BIBO3304的小鼠血清胰岛素水平显著升高(图1l,m),证实阻断Y1受体信号传导能够增强生理触发的胰岛素分泌。为了进一步测试这种药物干预是否可以改善胰岛移植的结果,我们使用最少数量的WT胰岛重复了移植实验,然后用BIBO3304口服一半的小鼠队列,另一半用安慰剂治疗。作为胰岛质量的对照,一组小鼠移植了最佳胰岛质量。接受安慰剂治疗的受体小鼠未能达到正常血糖,在整个实验过程中仍处于糖尿病状态(图2a,b)。相比之下,移植了少量WT胰岛并用BIBO3304治疗的小鼠迅速达到了正常血糖水平(图2a)。令人惊讶的是,当移植后第10天停止拮抗剂治疗时,这些小鼠能够维持正常血糖,直到第60天实验结束(图2a)。这表明,胰岛移植后早期对Y1受体信号传导的短暂抑制足以使葡萄糖稳态正常化。与血糖控制的改善一致,与安慰剂组相比,BIBO3304治疗组在移植后第5天的葡萄糖耐量也得到了显著改善(图2c,d)。一致地,BIBO3304治疗的小鼠也表现出优越的DIo,比安慰剂治疗的小鼠高12.28倍(DIo=0.066±0.0082,安慰剂组为0.819±0.324;每组4只小鼠)。与未经处理的胰岛移植物相比,经处理的BIBO3304的分析没有显示胰岛形态、凋亡、移植物血管化或内质网(ER)应激反应有任何明显差异(补充图3,4a),然而,经BIBO3304-处理的移植物中Ki67阳性β细胞的数量显著增加(图2e,f)。总之,这些数据表明,BIBO3304治疗小鼠血糖控制的改善是胰岛素分泌和胰岛增殖能力增加的结果,以应对生理葡萄糖水平的变化(图2e-h)[2]。 |
| 细胞实验 |
人Y1受体在幼仓鼠肾(BHK)细胞中稳定表达[1]
细胞在含有4.5 g/l葡萄糖、10%胎牛血清、1%PENStrep、1 mg ml71 g-418、1 mg ml 71潮霉素B的DMEM中生长。在受体结合试验前96小时,加入1 mM异丙基硫代半乳糖苷(IPTG)以诱导表达(Stratagene的Lac-Switch表达系统)。用0.06%EDTA/PBS去除融合细胞(孵育1分钟),并将其重新悬浮在15毫升孵育液中(MEM/25 mM HEPES+1%牛血清白蛋白,50 mM PMSF,0.1%杆菌肽,3.75 mM CaCl2)。在室温下离心10分钟(150 6 g)后,将沉淀物重新悬浮在50 ml培养基中,然后重新悬浮在30 ml培养基上。计数后,将细胞稀释至2.5 6 105个细胞ml71的®nal浓度。将200微升这种细胞悬浮液在室温下与30 pM[125I]NPY和增加浓度的试验化合物(10713±1074 M)一起孵育3小时,总体积为250 ml。在48℃下离心3000 6 g,10分钟后停止孵育。用0.25ml PBS重新离心沉淀物,并在g计数器中测量沉淀物。 表达大鼠Y1受体的人胚胎肾(HEK)293细胞[1] 用0.02%EDTA/PBS去除融合的细胞,并将其重新悬浮在10ml温育液中(MEM/25mM HEPES+0.5%BSA,50mM PMSF,0.1%杆菌肽,3.75mM CaCl2)。离心5分钟(150-6g)后,将沉淀物以等体积重新悬浮,并在10ml温育箱中进一步离心。将细胞稀释至1mio细胞ml71的浓度。将100ml该细胞悬浮液与30pM[125I]NPY溶液在室温下孵育3小时,并在250ml的总体积中增加试验化合物的浓度。按照大鼠下丘脑的描述停止孵育。 人Y5受体在HEK 293细胞中稳定转染[1] 按照BHK/Y1细胞的描述培养离心细胞,不同之处在于使用0.7mg ml71 G-418的浓度,不添加潮霉素,也不需要IPTG诱导。按照所述进行细胞培养和受体结合的孵育。最终浓度为550 H.A.Wieland等人的新型Y1受体拮抗剂BIBO 3304,其对喂食1.5 6 106个细胞ml71的影响,并按照Y1/BHK细胞的描述停止离心。 |
| 动物实验 |
Animal/Disease Models: Adult male Chbb:Thom rat, body weight 300 to 340 g [1]
Doses: 30 μg Route of Administration: Bilateral paraventricular nucleus injection Experimental Results: Hyperphagia after fasting was attenuated, especially before refeeding Within 2 hrs (hrs (hours)). Animal/Disease Models: 7weeks old C57BL/6JAusb mice[2] Doses: 0.5 μM Route of Administration: Oral Experimental Results:Serum insulin levels were Dramatically increased. Food-intake studies Adult male Chbb:Thom rats weighing between 300 and 340 g were individually housed and maintained on a 12 : 12 h lightdark cycle beginning at 06.00 h. Tap water and standard laboratory chow were available throughout except in the experiments where the animals were fasted for 24 h. After 1 week of habituation to their new housing conditions, the animals were anaesthetized with sodium pentobarbital (60 mg kg71 , i.p) for the placement of stainless steel guide cannulae. Bilateral guide cannulae (26 gauge) were placed 1 mm above the paraventricular nucleus according to the stereotaxic coordinates (Paxinos & Watson, 1986): AP:71.8, L:0.5, V:7.0. Guide cannulae were maintained in place on the skull with small metal screws and dental acrylic cement. Cannulae were closed with a stainless steel xstylet when not in use. Rats were allowed to recover for at least 1 week and were adapted to the injection procedure. On the day of the experiments drugs were injected between 08.00 and 09.00 h. Injection cannulae (33 gauge) were inserted 1 mm beyond the tips of the guide cannulae. The injection cannulae were attached by polyethylene tubing to a Hamilton microsyringe mounted in an infusion pump. Injection volume was 0.5 ml infused with a rate of 0.0125 ml s71 . In the ®rst set of experiments groups of 6 ± 12 rats received increasing doses (0.5 ± 32 mg, unilateral) of NPY receptor agonists into the PVN and food intake was monitored for at least 2 h. On the ®rst treatment day the groups were randomly assigned to the various doses. Rats had a wash-out period of at least 3 days between injections, after which the groups were randomized again to test the next agonist. Not more than 5 ± 6 injections were given in total. In the second set of experiments BIBO 3304 or its inactive enantiomer were given 10 min before the injection of dierent NPY receptor agonists, galanin or noradrenaline. All compounds were applied into the PVN and for each experiment 8 ± 22 rats were used and for each dose a dierent group of rats were used. In the last series of experiments BIBO 3304 was given to animals which were fasted for 24 h. Five minutes after bilateral PVN injection of BIBO 3304 the rats (n=12) were given free access to food and food intake was monitored for another 24 h.[1] Y1lox/lox mice were generated as previously described and crossed with mice expressing the Cre recombinase gene under the control of the rat insulin-2 promoter Tg(Ins2-cre)25Mgn/J(INS2cre/+) to generate Y1lox/lox/INS2cre/+ mice. INS2cre/+ mice were also crossed onto Gt(ROSA)26Sor tm9(EGFP/Rpl10a)Amc/J mice in order to produce β-cell specific expression of the EGFP–L10a fusion protein for mRNA isolation and qPCR analysis. For clarity, islet tissue from these mice is referred to as WT and Y1−/− respectively, throughout the text. Age-matched and sex-matched mice on a C57BL/6Ausb background were used for all experiments except stated otherwise. Female NOD mice were housed under a controlled temperature of 22 °C and a 12-hour light cycle (lights on from 0700 to 1900 hours) with ad libitum access to water and a standard chow diet (6% calories from fat, 21% calories from protein, 71% calories from carbohydrate, 14.0 MJ/kg). To avoid the stress caused by gavage, specific Y1 receptor antagonist BIBO 3304 was dissolved in distilled water and administrated daily in a form of jelly for the duration stated in the text. This method of drug delivery was developed in our lab and described previously31. In brief, we trained mice to voluntarily eat a vehicle jelly before the start of an experiment. After 2–5 days training, over 95% of mice consumed the entire portion of jelly (195 μl for a 25 g mouse) within 1 min of being placed in the cage and maintained a high avidity for jelly throughout the study period. At the commencement of an experiment, mice received BIBO 3304 containing jelly once per day for the time period indicated in each study, while control mice received vehicle jelly. [2] |
| 参考文献 | |
| 其他信息 |
Failure to secrete sufficient quantities of insulin is a pathological feature of type-1 and type-2 diabetes, and also reduces the success of islet cell transplantation. Here we demonstrate that Y1 receptor signaling inhibits insulin release in β-cells, and show that this can be pharmacologically exploited to boost insulin secretion. Transplanting islets with Y1 receptor deficiency accelerates the normalization of hyperglycemia in chemically induced diabetic recipient mice, which can also be achieved by short-term pharmacological blockade of Y1 receptors in transplanted mouse and human islets. Furthermore, treatment of non-obese diabetic mice with a Y1 receptor antagonist delays the onset of diabetes. Mechanistically, Y1 receptor signaling inhibits the production of cAMP in islets, which via CREB mediated pathways results in the down-regulation of several key enzymes in glycolysis and ATP production. Thus, manipulating Y1 receptor signaling in β-cells offers a unique therapeutic opportunity for correcting insulin deficiency as it occurs in the pathological state of type-1 diabetes as well as during islet transplantation.Islet transplantation is considered one of the potential treatments for T1DM but limited islet survival and their impaired function pose limitations to this approach. Here Loh et al. show that the Y1 receptor is expressed in β- cells and inhibition of its signalling, both genetic and pharmacological, improves mouse and human islet function. [2]
Since the Y1 receptor might be involved in an anxiolytic action (Wahlestedt et al., 1993; Kask et al., 1996) its antagonism could induce anxiogenic like side eects. In order to evaluate whether possible anxiogenic eects interfered with the BIBO 3304 mediated inhibition of feeding response the interaction between BIBO 3304 and the feeding response mediated by other stimulators, such as noradrenaline and galanin was examined. If anxiety plays a role in food intake inhibition mediated by BIBO 3304, noradrenaline and galanin induced food intake should also be inhibited. But since neither galanin nor noradrenaline induced food intake was inhibited a signi®cant anxiogenic component in the food intake inhibitory properties of BIBO 3304 can be ruled out. It has been published earlier that BIBP 3226 mediated eects on feeding antagonism is not due to general side eects in the experimental setting used (O'Shea et al., 1997; Kask et al., personal communication). However, our own data using BIBP 3226 were not as conclusive (Doods et al., 1996). Consequently, the inactive enantiomer to BIBO 3304 was tested in order to show that the food intake inhibition was not due to a general toxicity of structural components of BIBO 3304. Indeed, the enantiomer did not aect the feeding response. In the present study, the identi®cation of BIBO 3304, a nonpeptide compound that displays anity in the subnanomolar range and selectivity for the Y1 receptor subtype is shown. We hypothesize that the Y1 receptor plays an important role in NPY induced feeding and BIBO 3304 is a novel tool to study both food intake and other central eects mediated via the Y1 receptor. Moreover, the data presented with BIBO 3304 as well as with the agonists, e.g. [Leu31, Pro34]NPY indicate a complicated interplay between the dierent NPY receptors in feeding behaviour. [1] |
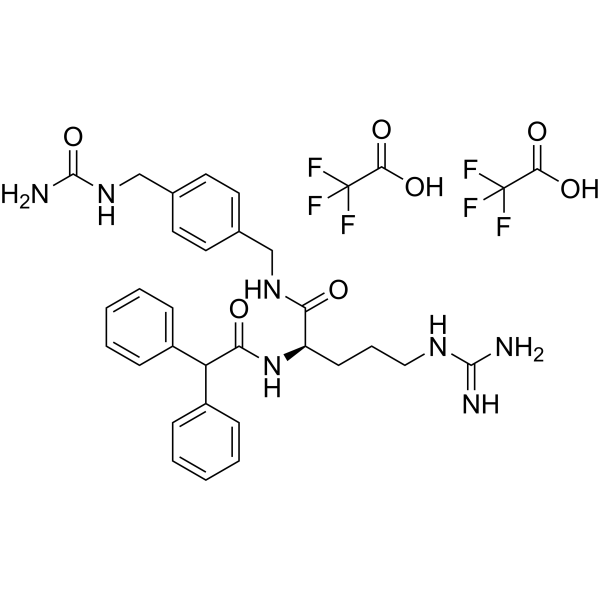
| 分子式 |
C33H37F6N7O7
|
|---|---|
| 分子量 |
757.68
|
| 精确质量 |
757.265
|
| CAS号 |
2310085-85-7
|
| PubChem CID |
122705981
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| tPSA |
252
|
| 氢键供体(HBD)数目 |
8
|
| 氢键受体(HBA)数目 |
14
|
| 可旋转键数目(RBC) |
13
|
| 重原子数目 |
53
|
| 分子复杂度/Complexity |
862
|
| 定义原子立体中心数目 |
1
|
| SMILES |
C1=CC=C(C=C1)C(C2=CC=CC=C2)C(=O)N[C@H](CCCN=C(N)N)C(=O)NCC3=CC=C(C=C3)CNC(=O)N.C(=O)(C(F)(F)F)O.C(=O)(C(F)(F)F)O
|
| InChi Key |
XWZMETGYCRXJJH-PPLJNSMQSA-N
|
| InChi Code |
InChI=1S/C29H35N7O3.2C2HF3O2/c30-28(31)33-17-7-12-24(26(37)34-18-20-13-15-21(16-14-20)19-35-29(32)39)36-27(38)25(22-8-3-1-4-9-22)23-10-5-2-6-11-23;2*3-2(4,5)1(6)7/h1-6,8-11,13-16,24-25H,7,12,17-19H2,(H,34,37)(H,36,38)(H4,30,31,33)(H3,32,35,39);2*(H,6,7)/t24-;;/m1../s1
|
| 化学名 |
(2R)-N-[[4-[(carbamoylamino)methyl]phenyl]methyl]-5-(diaminomethylideneamino)-2-[(2,2-diphenylacetyl)amino]pentanamide;2,2,2-trifluoroacetic acid
|
| 别名 |
BIBO-3304 TFA; BIBO3304 (diTFA); 2310085-85-7; CHEMBL5083453; BIBO 3304?;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
Typically soluble in DMSO (e.g. 10 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3198 mL | 6.5991 mL | 13.1982 mL | |
| 5 mM | 0.2640 mL | 1.3198 mL | 2.6396 mL | |
| 10 mM | 0.1320 mL | 0.6599 mL | 1.3198 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。