| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
| 靶点 |
Metabolic product of cystine
|
|---|---|
| 体外研究 (In Vitro) |
硫代牛磺酸是胱氨酸的代谢产物,含有一个可以以H(2)S形式释放的磺胺硫原子,这是一种对炎症反应具有调节活性的气体分子。通过测定人中性粒细胞中胱天蛋白酶-3的活性,评估了硫代牛磺酸对人白细胞自发凋亡的影响。添加100μM硫代牛磺酸可诱导55%的胱天蛋白酶-3活性抑制,类似于100μM H(2)S的抑制。有趣的是,在1mM GSH存在的情况下,观察到硫代牛磺酸对细胞凋亡的抑制作用增加。这些结果表明,谷胱甘肽可以调节硫代牛磺酸的生物活性,从而促进生成H(2)S和低牛磺酸的硫代磺酸盐的还原分解。由于硫代牛磺酸能够结合可逆还原的硫,因此认为这种硫代磺酸盐的生物合成可能是运输和储存H(2)S[1]的一种手段。
|
| 酶活实验 |
硫代牛磺酸是一种硫代磺酸盐化合物,在体液和组织中代谢产生一个硫原子。硫代牛磺酸是半胱氨酸代谢的有氧和无氧途径之间的相互连接分子。由于低牛磺酸和硫化物之间的反应而形成的硫代牛磺酸可以转化回H2S和低牛磺酸。因此,硫代牛磺酸可以被认为是一种安全、无毒的H2S储存形式,也是硫化物运输、储存和释放的生化途径中的重要关键中间体。硫烷含硫化合物有效地调节酶的活性并表现出抗氧化特性。有趣的是,硫代牛磺酸影响调节人类中性粒细胞功能反应的炎症过程,并对氧化损伤具有保护作用[2]。
|
| 细胞实验 |
硫代牛磺酸对人中性粒细胞自发凋亡的影响[1]
通过测量在37°C下预孵育3.5小时的中性粒细胞裂解物(5×106个细胞/mL)中的半胱氨酸天冬氨酸蛋白酶-3活性来评估自发凋亡。当在硫代牛磺酸(TTAU)存在的情况下进行预孵育步骤时,观察到胱天蛋白酶-3活性的浓度依赖性降低(图19.1)。由于硫代牛磺酸含有一个可以作为H2S释放的硫烷硫原子,因此还评估了NaHS对半胱氨酸天冬氨酸蛋白酶-3活性的影响。100μM硫代牛磺酸对半胱氨酸天冬氨酸蛋白酶-3活性的降低为55±3%,与100μM NaHS(57±3%)的降低相似。对照实验(未显示)表明,浓度在0.01至0.2 mM范围内的TTAU和NaHS均不影响重组半胱氨酸天冬氨酸蛋白酶-3的活性。 谷胱甘肽对硫代牛磺酸诱导的Caspase-3活性抑制的影响[1] 据报道,谷胱甘肽(GSH)通过影响半胱氨酸天冬氨酸蛋白酶-3活性来调节中性粒细胞凋亡(O'Neill等人,2000)。这种效果归因于其抗氧化活性(Wedi等人,1999)。为了深入了解TTAU的抑制机制,将这种硫代磺酸盐对半胱氨酸天冬氨酸蛋白酶-3活性的抑制作用与谷胱甘肽的抑制作用进行了比较(图19.2)。 |
| 参考文献 |
[1]. Adv Exp Med Biol. 2013:775:227-36. doi: 10.1007/978-1-4614-6130-2_19.
[2]. Adv Exp Med Biol. 2019:1155:755-771. doi: 10.1007/978-981-13-8023-5_66. |
| 其他信息 |
These results indicate that the thiosulfonate, thiotaurine, may exert regulatory effects on inflammation influencing lifespan of human neutrophils. Mature circulating neutrophils are constitutively committed to apoptosis. During inflammatory response, survival of neutrophils recruited into the inflamed area is significantly prolonged. Increased survival in the inflamed tissue permits neutrophils to fulfill their effector functions most efficiently. On the other hand, macrophage-mediated elimination of apoptotic neutrophils from the inflamed area has been recognized as a crucial mechanism for promoting resolution of inflammation (Savill and Fadok 2000; Simon 2003). It is recognized that the production of reactive oxygen species by activated cells accelerate the apoptosis and that superoxide release is required for spontaneous apoptosis (Ottonello et al. 2002; Scheel-Toellner et al. 2004). Moreover, the spontaneous and FAS-mediated apoptosis are prevented by antioxidants, such as GSH (Wedi et al. 1999). This effect has been ascribed to the ability of GSH to scavenge reactive oxygen species (Watson et al. 1997). It has been also shown that thiotaurine is highly effective in counteracting the damaging effect of oxidants (Acharya and Lau-Cam 2012). Thus, it is possible that the delay of spontaneous apoptosis of human neutrophils by thiotaurine may be related to its antioxidant activity. On the other hand, our results show that the inhibitory effect of thiotaurine on caspase-3 activity was higher than that of GSH. Moreover thiotaurine, in the presence of GSH, is more effective in influencing neutrophil apoptosis. These findings suggest that alternative or additional mechanisms of inhibition can be involved. It is well-known that GSH can act as a catalyst of the reductive breakdown of thiotaurine with generation of hypotaurine and H2S (Chauncey and Westley 1983). Accordingly, we found that human neutrophils generate H2S from thiotaurine with GSH as a necessary reductant in the reaction. It has been previously reported that H2S promotes the short-term survival of neutrophils by inhibition of caspase-3 cleavage (Rinaldi et al. 2006). Our results confirm the effect of H2S on prolonging the survival of neutrophils. Hence, it is likely that the sulfane sulfur of thiotaurine released as H2S in the presence of GSH, may contribute to the observed effect on neutrophil survival.[1]
The biological relevance of thiotaurine in mammalian is still a challenge to biochemical research. Biological roles have been sporadically reported (Costa et al. 1990; Baskin et al. 2000). On the contrary, in some marine organisms a key role for thiotaurine in the transport of sulfur has been strongly demonstrated (Pruski et al. 2001; Pruski and Fiala-Médioni 2003). Morevover, the metabolic origin of thiotaurine in mammalians is subject to debate, as is its fate. One pathway for thiotaurine metabolism is via transulfuration reactions with hypotaurine being the main intermediate (Cavallini et al. 1961; De Marco and Tentori 1961). These reactions can be spontaneous or catalyzed by sulfur transferases (De Marco et al. 1961; Chauncey and Westley 1983). Our experiments show that hypotaurine is the main metabolite of thiotaurine with a 1:1 stoichiometry, suggesting a role of thiotaurine as a biochemical intermediate in the transport, storage, and release of sulfide also in mammalians. This hypothesis is further supported by the fact that hypotaurine, present in leukocytes at millimolar concentration (Learn et al. 1990), can readily incorporate H2S formed during inflammation with production of thiotaurine (De Marco and Tentori 1961).[1] Since thiotaurine as well as hypotaurine, taurine, and H2S can modulate leukocyte functional responses, it would be worthy to investigate the metabolic and functional interplay between these sulfur compounds at inflammatory sites.[1] |
| 分子量 |
141.21248
|
|---|---|
| 精确质量 |
140.992
|
| CAS号 |
2937-54-4
|
| 相关CAS号 |
31999-89-0 (mono-hydrochloride salt);2937-54-4 (Parent)
|
| PubChem CID |
6858023
|
| 外观&性状 |
Solid
|
| 密度 |
1.541g/cm3
|
| 沸点 |
324.6ºC at 760mmHg
|
| 闪点 |
150.1ºC
|
| 蒸汽压 |
4.94E-05mmHg at 25°C
|
| 折射率 |
1.622
|
| LogP |
0.894
|
| tPSA |
93.35
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
7
|
| 分子复杂度/Complexity |
137
|
| 定义原子立体中心数目 |
0
|
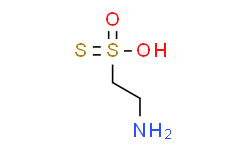
| SMILES |
NCCS(=S)(O)=O
|
| InChi Key |
SHWIJIJNPFXOFS-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C2H7NO2S2/c3-1-2-7(4,5)6/h1-3H2,(H,4,5,6)
|
| 化学名 |
2-hydroxysulfonothioylethanamine
|
| 别名 |
Thiotaurine; 2-hydroxysulfonothioylethanamine; 2-Aminoethanethiosulfonic acid; NQZ2D7AO62; Thiotaurine; 2937-54-4; 2-hydroxysulfonothioylethanamine; 2-Aminoethanethiosulfonic acid; NQZ2D7AO62; Sodium 2-aminosulphonothioacetate; EINECS 250-888-0; 31999-89-0;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 7.0817 mL | 35.4083 mL | 70.8165 mL | |
| 5 mM | 1.4163 mL | 7.0817 mL | 14.1633 mL | |
| 10 mM | 0.7082 mL | 3.5408 mL | 7.0817 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。