| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
浓度为 100、300 和 1000 µM 的二甲嗪会强烈抑制 HepG2 细胞活力 [1]。二甲嗪(3、10、30 µM;24 h)促进 HepG2 细胞的能量代谢从有氧三羧酸循环(TCA)和氧化磷酸化转变为无氧糖酵解[1]。在 HepG2 细胞中,异丙嗪(3、10、30 µM;24 小时)可抑制复合物 IV 活性 [1]。
|
|---|---|
| 体内研究 (In Vivo) |
三氯芬酸(46.3、139、417 mg/kg;口服;单次)在肾脏(18.64%)、脑(23.58%)、胃(21.94%)和肝脏(35.84%)中蓄积[1]。异丙嗪(46.3、139、417 mg/kg;口服;单剂量)比任何其他器官都更能提高小鼠肝脏和大脑中的 MDA 水平[1]。
|
| 细胞实验 |
RT-PCR[1]
细胞类型: HepG2 细胞 测试浓度: 3、10、30 µM 孵育时间: 24 小时 实验结果: 30 µM 时,乳酸脱氢酶 B (LDHB) 水平显着增加,6-磷酸果糖-2-激酶/果糖-2,6-二磷酸酶略有增加3(PFKFB3)。在 3、10 和 30 µM 噻嗪酮处理下,ATP 水平以浓度依赖性方式分别降低至载体对照水平的 91.3%、87.9 和 67.2%。乳酸水平显着增加。 免疫荧光[1] 细胞类型: HepG2 细胞 测试浓度: 3、10、30 µM 孵育时间: 24 小时 实验结果: 在 3、10 和 30 ℃ 进行噻嗪酮处理后,复合物 IV 的活性显着抑制至载体对照水平的 82.2、69.2 和 63.4%分别为μM。 细胞活力测定[1] 细胞类型: HepG2 细胞 测试浓度: 3、10、30 µM <孵育时间: 24 小时 实验结果: 以不依赖浓缩物的方式显着提高细胞内 ROS 水平,并降低 mtDNA 含量。 |
| 动物实验 |
Animal/Disease Models: Male C57BL/6 mice (6 to 8weeks old)[1].
Doses: 46.3, 139, 417 mg/kg Route of Administration: Oral administration; single Experimental Results: Tended to elevate the MDA level in all organs, and the most significant concentration-dependent increases were observed in the liver and brain. demonstrated the highest concentrations in the liver (35.84%) followed by the brain (23.58%), stomach (21.94%) and kidney (18.64%), while the levels in the mouse spleen and heart were below the limit of detection. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
(14)C-buprofezin (radiochemical purity >97%) administered by gavage to 5 rats/sex/dose at 10 and 100 mg/kg; in males, 90-91% of dose eliminated by 48 hr (20-21% in urine, 69-71% in feces); in females, 87-89% of dose eliminated by 48 hr (13-14% in urine, 73-76% in feces); elimination faster in males during 1st 24 hr, but equalized by 48 hr; <1% of dose remained in body by 7 days; > or =30% of male dose and > or =38% of female dose recovered in bile at 24 hr; chromatography of urine, bile, and feces indicated extensive conjugation; bile cannulation of 3M/3F revealed that fecal metabolites were likely of bile origin. [(14)C-phenyl]-buprofezin (2 or 22.5 mCi/mmol; >97% radiochemical purity) suspended in 1 mL of olive oil was administered by gavage to fasted /rats/ at 10 and 100 mg/kg (number of animals varied with the experiment); over 90% of administered dose was excreted by 48 hr at both concentrations; by 96 hr at both concentrations, 70-74% of dose excreted in feces (though a delay at the high dose relative to the low dose was noted through 24 hr), 21-25% in urine, very low amounts excreted as expired (14)C-CO2; at 10 mg/kg, 12% of the parent compound was excreted into the feces; Cmax in blood occurred at 9 hr for both doses after which concentrations declined biphasically (t1/2 =13 and 60 hr); peak levels of radiolabel occurred in tissues at 5-9 hr post dose, after which tissue levels decreased biphasically with a t1/2 of 3.5-15 hr and 15-72 hr for the 2 phases; by 96 hr tissue residue levels were low. Male rats /were/ fed diet containing buprofezin at 200 or 1000 ppm for up to 24 weeks; 3/dose were sacrificed on days 2 and 4 and on weeks 1, 2, 4, 8, 12, 16, and 24; buprofezin levels measured by gas-liquid chromatography after extraction from blood, brain, liver, kidney, adipose tissue, and muscle; detection limit: 0.1 ppm; 200 ppm: only adipose tissue attained levels high enough to consistently detect, remaining stable between 4 days and 24 weeks at a mean concentration of 0.43-1.10 ppm; an occasional animal showed detectable levels in liver, while kidney, muscle, and brain never showed detectable levels; 1000 ppm: adipose tissue peaked at 10.53 ppm on day 4, declining to 3.40 ppm at 24 weeks; liver retained a stable concentration of 0.21-0.96 over the entire period; kidneys were near or below the detection limit for 8 weeks and undetectable thereafter; brain was near or below the detection limit for 1 week and undetectable thereafter; muscle was near or below the detection limit for 2 weeks and undetectable thereafter (no measurement at 4 weeks); test article thus did not accumulate in any tissue at either concentration. Metabolism / Metabolites [(14)C-phenyl]-buprofezin (2 or 22.5 mCi/mmol; >97% radiochemical purity) suspended in 1 mL of olive oil was administered by gavage to fasted /rats/ at 10 and 100 mg/kg (number of animals varied with the experiment); ... metabolite studies revealed hydroxylation of the phenyl ring, oxidation of sulfur, and cleavage of the thiadiazin ring, with evidence of glucuronic and sulfuric conjugation. /In/ ruminants: 2-tert-butylimino-5-(4-hydroxyphenyl)-3-isopropyl-1,3,5- thiadiazinan-4-one (BF2) was the major residue identified in liver and kidney (residue in fat and muscle were < or =0.020 ppm) and N-(4-hydroxyphenyl)acetamide (BF23) was the major residue identified in milk from the ruminant metabolism study (all other residue were <10% total radioactive residue (TRR); 3.5x maximum theoretical dietary burden (MTDB)). ...The residues of concern in milk are buprofezin and BF23; ... the residues of concern in ruminant tissues are buprofezin and BF2. |
| 参考文献 |
|
| 其他信息 |
Description
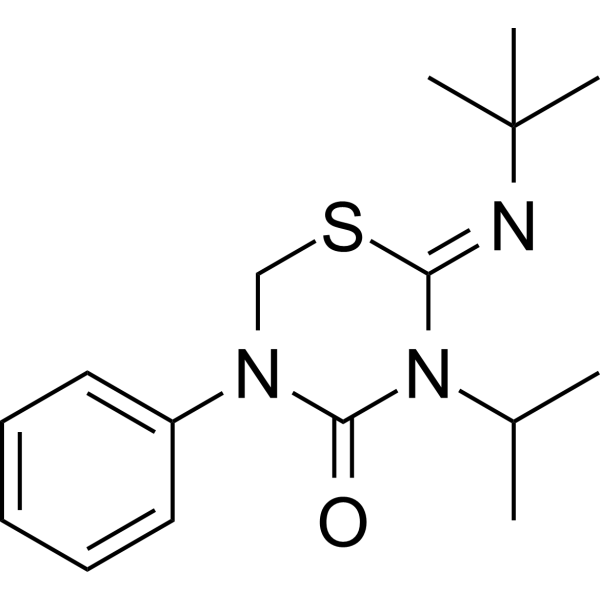
Buprofezin is a 2-(tert-butylimino)-5-phenyl-3-(propan-2-yl)-1,3,5-thiadiazinan-4-one in which the C=N double bond has Z configuration. It has a role as an insecticide and a member of homopteran inhibitor of chitin biosynthesis. |
| 分子式 |
C16H23N3OS
|
|---|---|
| 分子量 |
305.44
|
| 精确质量 |
305.156
|
| CAS号 |
69327-76-0
|
| 相关CAS号 |
Buprofezin-d6;2140803-94-5
|
| PubChem CID |
50367
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.18
|
| 沸点 |
273°C (12 torr)
|
| 熔点 |
104-106°C
|
| 闪点 |
176-178°C
|
| 折射率 |
1.52-1.522
|
| LogP |
4.185
|
| tPSA |
61.21
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
21
|
| 分子复杂度/Complexity |
408
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
PRLVTUNWOQKEAI-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C16H23N3OS/c1-12(2)19-14(17-16(3,4)5)21-11-18(15(19)20)13-9-7-6-8-10-13/h6-10,12H,11H2,1-5H3
|
| 化学名 |
2-tert-butylimino-5-phenyl-3-propan-2-yl-1,3,5-thiadiazinan-4-one
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 100 mg/mL (327.40 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.18 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (8.18 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (8.18 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.2740 mL | 16.3698 mL | 32.7397 mL | |
| 5 mM | 0.6548 mL | 3.2740 mL | 6.5479 mL | |
| 10 mM | 0.3274 mL | 1.6370 mL | 3.2740 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。