| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mL |
|
||
| 25mL |
|
||
| 50mL |
|
||
| Other Sizes |
|
| 体内研究 (In Vivo) |
肝毒性作用与 N,N-二甲基甲酰胺(DMF;N-甲酰二甲胺)有关[3]。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The solvent can pass through the intact skin or can be absorbed through the lungs. Dimethylformamide reached an average level of 2.8 ug/L in the blood of subjects exposed to 21 ppm of the vapor for 4 hr, and was undetectable at 4 hr after the exposure; the metabolite, methylformamide, averaged between 1 and 2 mg/L in the blood and this level was maintained for at least 4 hr after exposure. Maximal blood levels of about 14 and 8 ug/L were observed for dimethylformamide and methylformamide, respectively, at 0 and 3 hr, after a 4 hr exposure to 87 ppm of the vapor. Repeated daily exposures to 21 ppm of dimethylformamide did not result in accumulation of the chemical or its metabolite in blood. /Dimethylformamide and methylformamide/ Eight healthy male subjects were exposed to dimethylformamide (DMF) vapor at a concn of 8.79 + or - 0.33 ppm for 6 hr daily for 5 consecutive days. All urine voided by the subjects was collected from the beginning of the first exposure to 24 hr past the end of the last exposure and each sample was analyzed for monomethylformamide. Monomethylformamide was rapidly eliminated from the body with urine values peaking within a few hours following the end of each exposure period. The mean for the 7 hr (end of exposure) sample was 4.74 mg/mL. The amount of N-methylformamide recovered in the urine represents only 2-6% of the dose of dimethylformamide inhaled. A substantial portion of an absorbed dose of DMF is excreted unchanged in the expired breath. The urinary concn of N-methylformamide is probably the best index of worker exponent dimethylformamide. For more Absorption, Distribution and Excretion (Complete) data for N,N-DIMETHYLFORMAMIDE (12 total), please visit the HSDB record page. Metabolism / Metabolites N,N-dimethylformamide (DMF) is metabolized by the microsomal cytochrome p-450 into mainly N-hydroxymethyl- N-methylformamide (HMMF), which further breaks down to N-methyformamide (NMF). However, the detailed mechanism of its toxicity remains unclear. We investigated the metabolism and the toxicity of DMF using the isolated perfused liver model. DMF was added to the recirculating perfusate of the isolated perfused rat liver at concentrations of 0, 10 and 25 mM. Samples were collected from the inferior vena cava at 0, 30, 45, 60, 75, and 90 minutes following addition of the DMF. The metabolites of DMF were analyzed using Gas-chromatography (GC). The changes in the rate of oxygen consumption by the DMF were monitored during perfusion. The enzyme activities (aspartic aminotransferase:AST, alanine aminotransferase:ALT, and lactic dehydrogenase:LDH)) in the perfusate were monitored to see if DMF caused hepatotoxicity. As the perfusion progressed, the DMF concentration in the perfusate decreased, but the level of NMF increased to a maximum of 1.16 mM. The rate of oxygen consumption increased at DMF concentrations of 10 mM and 25 mM. However, when a known inhibitor of cytochrome P-450, SKF 525A (300 uM), was used to pretreat the perfusate prior to the addition of the DMF, the rate of oxygen consumption was significantly inhibited, indicating the cytochrome P-450 system was responsible for the conversion of DMF to NMF. On addition of the DMF, the activities of the enzymes AST, ALT and LDH were significantly increased a time and dose dependent manner. However, following pretreatment with SKF 525A, their releases were inhibited. ... Two groups of workers investigated metab of DMF on volunteers. ... Both found that majority of absorbed substance is eliminated within 24 hr and that main urinary metabolite is n-methyl formamide. Its concn was related to intensity of exposure. It is known that dimethylformamide is metabolized in man by sequential N-demethylation to methylformamide and formamide, which are largely eliminated in the urine. Blood and urine samples of rats and dogs which had been exposed to DMF were examined by GLC analysis and N-methylformamide(NMF) and formamide were detected in addition to DMF. These metabolites were eliminated faster in rats than in dogs. It has been suggested recently that the major metabolite of DMF which has been characterized an NMF by GLC is no NMF but N-hydroxymethyl-N-methylformamide (HMMF). HMMF is the immediate product of methyl C-hydroxylation of DMF and is a relatively stable carbinolamide in aqueous soln. It is, however thermally labile so that it decomposes quantitatively to NMF and presumably formaldehyde on the GLC column. The evidence that the metabolite which has been characterized as NMF is really HMMF is based on three studies. /One study/ found a formaldehyde precursor in the urine of mice which had received DMF. This metabolite liberated formaldehyde only after alkaline hydrolysis. In aqueous soln, authentic HMMF also decomposed to formaldehyde only on alkaline hydrolysis. /Another study/ isolated a urinary metabolite of DMF in rats by HPLC and subjected it to mass spectrometric analysis. The observed fragmentation pattern suggested the presence of HMMF, even though the mass fragments, including the one corresponding to the molecular ion, were also detected in control urine samples. Unequivocal evidence for the contention that HMMF and not NMF is the major metabolite of DMF was recently obtained by high-field proton NMF spectroscopy of urine samples of mice which had received DMF. HMMF exists in 2 rotameric forms and the methyl and formyl protons in the two rotamers are not equivalent. The resonance frequencies corresponding to the methyl and formyl protons of both rotamers were prominent signals in the NMR spectrum of the urine. However, at the resonance frequency of the methyl protons of NMF only a minute signal was observed. In this study dimethylamine and methylamine were found to be minor urinary metabolites of DMF in mice. For more Metabolism/Metabolites (Complete) data for N,N-DIMETHYLFORMAMIDE (14 total), please visit the HSDB record page. Dimethyl formamide may be absorbed following ingestion, inhalation, and dermal exposure, and is distributed evenly throughout the body. Metabolism takes place in the liver via microsomal enzyme systems, producing N-hydroxymethyl- N-methylformamide (DMF-OH) as the main urinary metabolite. Biological Half-Life Whole body: 4 hours; [TDR, p. 551] |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Dimethylformamide ... is an organic solvent produced in large quantities through-out the world. It is used in the chemical industry as a solvent, an intermediate & an additive. Dimethylformamide is a colorless liquid with an unpleasant slight odor that ... has poor warning properties & individuals may be exposed through the inhalation of vapor. Occupational exposure occurs via skin contact with dimethylformamide liquid & vapors. ... Toxic amounts of dimethylformamide may be absorbed by inhalation & through the skin. Absorbed dimethylformamide is distributed uniformily. The /metabolism/ of dimethylformamide takes place mainly in the liver, with the aid of microsomal enzyme systems. In animals & humans, the main product of dimethylformamide biotransformation is N-hydroxymethyl-N-methylformamide. This metabolite is converted during gas chromatographic analysis to N-methylformamide, which itself (together with N-hydroxymethylformamide & formamide) a minor metabolite. ... In metabolic studies & biological monitoring, urinary concentration are expressed as N-hydroxymethylformamide. ... The determination of the /metabolites/ ... in the urine may be a suitable biological indicator of total dimethylformamide exposure. In experimental animals, it has been demonstrated that dimethylformamide metabolism is saturated at high levels &, at very high levels, dimethylformamide inhibits its own metabolism. Metabolic interaction occurs between dimethylformamide & ethanol. ... The effects of dimethylformamide on the environment have not been well studied. The toxicity for aquatic organisms appears to be low ... The acute toxicity of dimethylformamide in a variety of species is low ... . It is a slight to moderate skin & eye irritant. One study on guinea pigs indicated no sensitization potential. Dimethylformamide can facilitate the absorption of other chemical substances through the skin. Exposure of experimental animals to dimethylformamide via all routes of exposure may cause dose related liver injury. ... In some studies, signs of toxicity in the myocardium & kidneys have been /noted/. Dimethylformamide was ... found to be inactive, both in vitro & in vivo, in an extensive set of short term tests for genetic & related effects. No adequate long term carcinogenicity studies on experimental animals have been reported. ... Skin irritation & conjunctivitis have been reported after direct contact with dimethylformamide in /humans/. After accidental exposure to high levels of /this cmpd/, abdominal pain, nausea, vomiting, dizziness & fatigue occur within 48 hr. Liver function may be disturbed, & blood pressure changes, tachycardia & ECG abnormalities have been reported. ... Following long-term repeated exposure, symptoms include headache, loss of appetite & fatigue. Biochemical signs of liver dysfunction may be observed. Exposure to dimethylformamide, even at concn below 30 mg/cu m may cause alcohol intolerance. Symptoms may include a sudden facial flush, tightness of the chest, & dizziness sometimes accompanied by nausea & dypsnea. ... There is limited evidence that dimethylformamide is carcinogenic for human beings. An incr in testicular tumors was reported in one study, whereas another study showed incr incidence of tumors of the buccal cavity & pharynx, but not the testes. In two studies with limited details, an incr frequency of miscarriages was reported in women exposed to dimethylformamide among other chemicals. While the mechanism of action of dimethyl formamide has not bee fully elucidated, thiocarbamate pesticides have been shown to inhibit aldehyde dehydrogenases. (A2459) Toxicity Data LCLo (rat) = 5,000 ppm/6 hr LD50: 2800 mg/kg (Oral, Rat) (T14) LD50: 1400 mg/kg (Intraperitoneal, Rat) (T14) LD50: 3800 mg/kg (Subcutaneous, Rat) (T14) LD50: 2000 mg/kg (Intravenous, Rat) (T14) Interactions In acetone pretreated male CD 1 mice, dimethy1formamide, given as a single ip dose of 1000 mg/kg bw, resulted in liver necrosis and a strong incr in serum alanine aminotransferase activity. In contrast, no signs of hepatotoxicity were found in non pretreated mice given the same dose or in pretreated or nonpretreated male Sprague Dawley rats given up to 2000 mg/kg bw as a single ip dose. These differences are probably related to the highly different substrate affinities of CYP2EI in rats and mice. In a study of 102 workers, 19 had experienced manifestation of alcohol intolerance, among them facial flushing, sweating, dizziness, and palpitation, mainly within 24 hr of exposure /to DMF/. Of the 34 episodes recorded, 26 occurred after the workers had consumed alcoholic drinks. Among the work population that included a total of 13 workers, seven had abdominal colic which was sustained for more than 3 days, three had abnormal liver function, and two had facial flushing. /DMF (Dimethylformamide) exposure/ A veterinary euthanasia drug containing embutramide, mebezonium, tetracaine, and dimethylformamide (DMF; T-61 or Tanax) may cause serious manifestations or even fatalities after self-poisoning. Immediate toxicity is mainly due to a general anesthetic and due to a neuromuscular blocking agent, while delayed hepatotoxicity seems related to the solvent DMF. The protective role of N-acetylcysteine (NAC) administration remains debatable. Two male veterinarians (50- and 44-year-old) attempted suicide by injecting T-61 in the precordial area for the first one, and by ingesting 50 mL for the second. Both received NAC (for 14 days in the first case and only for 20 hr in the second). Urine was collected for the serial determination of DMF, N-methylformamide (NMF), and N-acetyl-S-(N-methylcarbamoyl)cysteine (AMCC). Both patients developed only mild signs of liver injury. The metabolite of DMF, NMF, appeared rapidly in the urine, while a further delay was necessary for AMCC excretion. The kinetics of elimination of DMF and DMF metabolites were slightly slower than those reported in exposed workers. While both patients had a favorable outcome, there is no clear evidence that NAC could directly influence NMF and AMCC excretion... For more Interactions (Complete) data for N,N-DIMETHYLFORMAMIDE (12 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 MOUSE IP 1120 MG/KG (1.2 ML/KG) LD50 Mouse oral 6.8 mL/kg LD50 Swiss mouse ip 3.07 g/kg daily for 21 days LD50 Tumor bearing BDF1 mouse ip 1.23 g/kg daily for 9 days For more Non-Human Toxicity Values (Complete) data for N,N-DIMETHYLFORMAMIDE (30 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
N,N-Dimethylformamide can cause cancer according to California Labor Code and the World Health Organization's International Agency for Research on Cancer (IARC).
N,n-dimethylformamide appears as a water-white liquid with a faint fishy odor. Flash point 136 °F. Slightly less dense than water. Vapors heavier than air. Toxic by inhalation or skin absorption. May irritate eyes. N,N-dimethylformamide is a member of the class of formamides that is formamide in which the amino hydrogens are replaced by methyl groups. It has a role as a polar aprotic solvent, a hepatotoxic agent and a geroprotector. It is a volatile organic compound and a member of formamides. It is functionally related to a formamide. Dimethylformamide is used as an industrial solvent and in the production of fibers, films, and surface coatings. Acute (short-term) exposure to dimethylformamide has been observed to damage the liver in animals and in humans. Symptoms of acute exposure in humans include abdominal pain, nausea, vomiting, jaundice, alcohol intolerance, and rashes. Chronic (long-term) occupational exposure to dimethylformamide by inhalation has resulted in effects on the liver and digestive disturbances in workers. Human studies suggested a possible association between dimethylformamide exposure and testicular cancer, but further studies failed to confirm this relationship. EPA has not classified dimethylformamide with respect to its carcinogenicity. N,N-Dimethylformamide has been reported in Nicotiana tabacum and Cystoseira barbata with data available. N,N-Dimethylformamide (DMF) is a clear liquid that has been widely used in industries as a solvent, an additive, or an intermediate because of its extensive miscibility with water and most common organic solvents. Its health effects include hepatotoxicity and male reproductoxicity, possibly linked with mitochondrial DNA (mtDNA) alterations including mtDNA common deletion (delta-mtDNA4977) and mtDNA copy number; during the biotransformation of DMF in the body, free radicals are formed, including hydroxyl radicals. The world-wide consumption of DMF in 2001 was approximately 285,000 metric tonnes and most of that was used as an industrial solvent. Overexposure to DMF could result in hepatotoxicity, alcohol intolerance, possible embryotoxicity and teratogenicity in humans and animals, and decline of human sperm motility. Based on its wide application and a wide range of toxic effects, DMF has been selected as one of the four priority compounds for human field studies by the National Toxicology Program (NTP) of the US National Institute of Environmental Health Sciences (NIEHS). The current permissible exposure limit for DMF in the working environment is 10 ppm in both USA and Taiwan. The concentrations of two major DMF metabolites in urine, N-methylformamide (U-NMF) of 15 mg/L and N-acetyl-S-(N-methylcarbamoyl) cysteine (U-AMCC) of 40 mg/L, were recommended as the biological exposure indices (BEIs) by the American Conference of Governmental Industrial Hygienists for DMF exposure in workplace. (A7735). N,N-dimethylformamide is a metabolite found in or produced by Saccharomyces cerevisiae. A formamide in which the amino hydrogens are replaced by methyl groups. Mechanism of Action N,N-Dimethylformamide (DMF) is an organic solvent extensively used in industries such as synthetic leather, fibers and films, and induces liver toxicity and carcinogenesis. Despite a series of experimental and clinical reports on DMF-induced liver failure, the mechanism of toxicity is yet unclear. This study investigated whether DMF in combination with a low dose of hepatotoxicant enhances hepatotoxicity, and if so, on what mechanistic basis. Treatment of rats with either DMF (50-500 mg/kg/day, for 3 days) or a single low dose of CCl(4) (0.2mL/kg) alone caused small increases in plasma transaminases and lactate dehydrogenase activities. However, combinatorial treatment of DMF with CCl(4) markedly increased blood biochemical changes. Histopathology confirmed the synergism in hepatotoxicity. Moreover, DMF+CCl(4) caused PARP cleavage and caspase-3 activation, but decreased the level of Bcl-xL, all of which confirmed apoptosis of hepatocytes. Consistently, DMF+CCl(4) treatment markedly increased lipid peroxidation. By contrast, treatment of DMF in combination with lipopolysaccharide, acetaminophen or d-galactosamine caused no enhanced hepatotoxicity. Given the link between endoplasmic reticulum (ER) dysfunction and cell death, ER stress response was monitored after DMF and/or CCl(4) treatment. Whereas either DMF or CCl(4) treatment alone marginally changed the expression levels of glucose-regulated protein 78 and 94 and phosphorylated PKR-like ER-localized eIF2alpha kinase, concomitant treatment with DMF and CCl(4) synergistically induced them with increases in glucose-regulated protein 78 and C/EBP homologous protein mRNAs. /These/ results demonstrate that DMF treatment in combination with CCl(4) synergistically increases hepatocyte death, which may be associated with the induction of severe ER stress. |
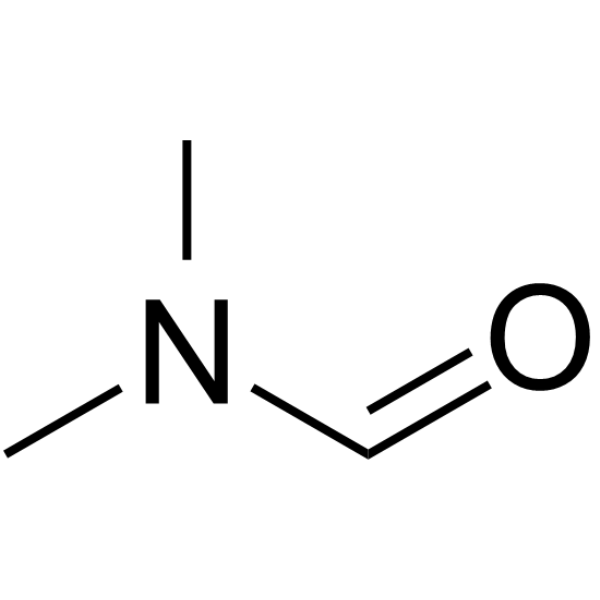
| 分子式 |
C3H7NO
|
|---|---|
| 分子量 |
73.09
|
| 精确质量 |
73.052
|
| CAS号 |
68-12-2
|
| PubChem CID |
6228
|
| 外观&性状 |
Colorless to light yellow liquid
|
| 密度 |
0.948 g/mL at 20 °C
|
| 沸点 |
153 °C(lit.)
|
| 熔点 |
-61 °C
|
| 闪点 |
136 °F
|
| 折射率 |
n20/D 1.430(lit.)
|
| LogP |
0.34
|
| tPSA |
20.31
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
0
|
| 重原子数目 |
5
|
| 分子复杂度/Complexity |
33.9
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CN(C)C=O
|
| InChi Key |
ZMXDDKWLCZADIW-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C3H7NO/c1-4(2)3-5/h3H,1-2H3
|
| 化学名 |
N,N-dimethylformamide
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 100 mg/mL (1368.18 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 13.6818 mL | 68.4088 mL | 136.8176 mL | |
| 5 mM | 2.7364 mL | 13.6818 mL | 27.3635 mL | |
| 10 mM | 1.3682 mL | 6.8409 mL | 13.6818 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。