| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 50g |
|
||
| 100g |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
在全细胞膜片钳记录中,5-HT3 钳传导的内向电流被普鲁卡因 (0.01-100 μM) 抑制。普鲁卡因的 KD 为 1.7 μM,似乎对 5-HT3 产生互补抑制作用 [1]。普鲁卡因是一种 DNA 甲基化剂,利用毛细管快速或高效酶消化整个 DNA,将 5-甲基胞嘧啶 DNA 的量减少 40%。此外,重甲基化的 CpG 岛可以被普鲁卡因去甲基化。这些杏仁同样会受到丙帕辛的生长抑制作用,导致有丝分裂曼哈顿分裂 [2]。普鲁卡因改变杏仁核输出的一部分的突触传递并兴奋边缘系统中的细胞[3]。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
With normal kidney function, the drug is excreted rapidly by tubular excretion. /PARA-AMINOBENZOIC ACID/ ... IS EXCRETED IN URINE TO EXTENT OF ABOUT 80%, EITHER UNCHANGED OR IN CONJUGATED FORM. ONLY 30% OF DIETHYLAMINOETHANOL CAN BE RECOVERED IN URINE; REMAINDER UNDERGOES METABOLIC DEGRADATION. ... IT HAS BEEN SHOWN THAT, FOLLOWING INTRODUCTION OF PROCAINE INTO EPIDURAL SPACE IN USUAL ANESTHETIC DOSE, SIGNIFICANT CONCN IS ACHIEVED IN SPINAL FLUID. RATE OF ENZYMATIC HYDROLYSIS OF LOCAL ANESTHETIC AGENTS BY SPINAL FLUID IS SLOW. ... DURATION OF ANESTHESIA DEPENDS UPON RATE AT WHICH DRUG IS REMOVED FROM CSF & FROM NERVE ROOTS WHERE IT EXERTS ITS ACTION. /LOCAL ANESTHETICS/ ABSORPTION DOES NOT TAKE PLACE FROM STOMACH, ESOPHAGUS, OR BLADDER IF MUCOSA INTACT. /LOCAL ANESTHETICS/ For more Absorption, Distribution and Excretion (Complete) data for PROCAINE (7 total), please visit the HSDB record page. Metabolism / Metabolites Hydrolysis by plasma esterases to PABA REDUCED RATE OF PROCAINE HYDROLYSIS IN SERUM FROM UREMIC PT IS DUE TO DECR ENZYME ACTIVITY RATHER THAN COMPETITIVE INHIBITION OF ENDOGENOUS SUBSTANCE IN SERUM. ... /Procaine/ is hydrolyzed in vivo to produce paraaminobenzoic acid ... YIELDS 2-DIETHYLAMINOETHYL P-ACETAMIDOBENZOATE IN GUINEA PIG; BILLIAR, RB, & EIK-NES, KB, ARCHS BIOCHEM BIOPHYS, 115, 318 (1966). /FROM TABLE/ Hydrolysis by plasma esterases to PABA Route of Elimination: With normal kidney function, the drug is excreted rapidly by tubular excretion. Half Life: 7.7 minutes Biological Half-Life 7.7 minutes SERUM T/2 VALUES FOR PROCAINE WERE LONGER FOR PT WITH LIVER DISEASE (2.3 MIN), PT WITH RENAL FAILURE (1.4 MIN), & NEW-BORN INFANTS (1.4 MIN) THAN FOR HEALTHY ADULTS (0.66 MIN). INFUSION OF 2% PROCAINE-HCL ADMIN TO 12 WOMEN UNDERGOING HYSTERECTOMY. STEADY-STATE PLASMA LEVELS WERE ACHIEVED WITHIN 20-30 MIN AFTER COMMENCEMENT OF INFUSION. AFTER TERMINATION OF INFUSION DISTRIBUTION T/2 OF 2.49 MIN & ELIMINATION T/2 OF 7.69 MIN. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation The term "Novocaine" (procaine) is often equated to local anesthetic. The exact identity of any local anesthetic should be verified, especially since procaine is no longer marketed in in the US as a single ingredient product. No information is available on the use of procaine during breastfeeding. Based on the short half-life of procaine in the plasma and the low excretion of other local anesthetics into breastmilk, a single dose of procaine during breastfeeding is unlikely to adversely affect the breastfed infant. However, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| 参考文献 |
|
| 其他信息 |
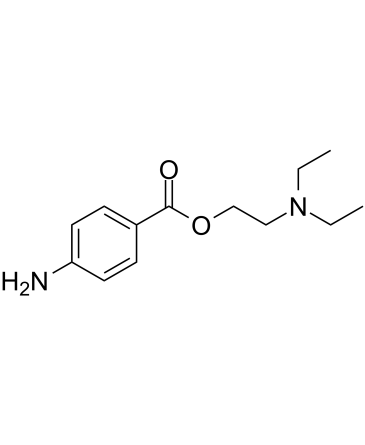
Procaine is a benzoate ester, formally the result of esterification of 4-aminobenzoic acid with 2-diethylaminoethanol but formed experimentally by reaction of ethyl 4-aminobenzoate with 2-diethylaminoethanol. It has a role as a local anaesthetic, a central nervous system depressant, a peripheral nervous system drug and a drug allergen. It is a benzoate ester, a substituted aniline and a tertiary amino compound. It is functionally related to a 2-diethylaminoethanol and a 4-aminobenzoic acid. It is a conjugate base of a procaine(1+).
A local anesthetic of the ester type that has a slow onset and a short duration of action. It is mainly used for infiltration anesthesia, peripheral nerve block, and spinal block. (From Martindale, The Extra Pharmacopoeia, 30th ed, p1016). Procaine has also been investigated as an oral entry inhibitor in treatment-experienced HIV patients. Procaine is a benzoic acid derivative with local anesthetic and antiarrhythmic properties. Procaine binds to and inhibits voltage-gated sodium channels, thereby inhibiting the ionic flux required for the initiation and conduction of impulses. In addition, this agent increases electrical excitation threshold, reduces rate of rise of action potential and slows nerve impulse propagation thereby causing loss of sensation. Procaine is only found in individuals that have used or taken this drug. It is a local anesthetic of the ester type that has a slow onset and a short duration of action. It is mainly used for infiltration anesthesia, peripheral nerve block, and spinal block. (From Martindale, The Extra Pharmacopoeia, 30th ed, p1016). [PubChem]Procaine acts mainly by inhibiting sodium influx through voltage gated sodium channels in the neuronal cell membrane of peripheral nerves. When the influx of sodium is interrupted, an action potential cannot arise and signal conduction is thus inhibited. The receptor site is thought to be located at the cytoplasmic (inner) portion of the sodium channel. Procaine has also been shown to bind or antagonize the function of N-methyl-D-aspartate (NMDA) receptors as well as nicotinic acetylcholine receptors and the serotonin receptor-ion channel complex. A local anesthetic of the ester type that has a slow onset and a short duration of action. It is mainly used for infiltration anesthesia, peripheral nerve block, and spinal block. (From Martindale, The Extra Pharmacopoeia, 30th ed, p1016). See also: Procaine Hydrochloride (has salt form); Penicillin G Procaine (is active moiety of); Procaine Merethoxylline (has salt form) ... View More ... Drug Indication Used as a local anesthetic primarily in oral surgery Mechanism of Action Procaine acts mainly by inhibiting sodium influx through voltage gated sodium channels in the neuronal cell membrane of peripheral nerves. When the influx of sodium is interrupted, an action potential cannot arise and signal conduction is thus inhibited. The receptor site is thought to be located at the cytoplasmic (inner) portion of the sodium channel. Procaine has also been shown to bind or antagonize the function of N-methyl-D-aspartate (NMDA) receptors as well as nicotinic acetylcholine receptors and the serotonin receptor-ion channel complex. LOCAL ANESTHETICS BLOCK CONDUCTION BY DECREASING OR PREVENTING THE LARGE TRANSIENT INCREASE IN THE PERMEABILITY OF EXCITABLE MEMBRANES TO SODIUM IONS THAT NORMALLY IS PRODUCED BY A SLIGHT DEPOLARIZATION OF THE MEMBRANE. /LOCAL ANESTHETIC/ LOCAL ANESTHETICS BLOCK CONDUCTION IN NERVE PERHAPS BY COMPETING WITH CALCIUM @ SOME SITE THAT CONTROLS PERMEABILITY OF MEMBRANE. ... LOCAL ANESTHETICS ALSO REDUCE PERMEABILITY OF RESTING NERVE TO POTASSIUM AS WELL AS TO SODIUM IONS. /LOCAL ANESTHETICS/ AS THE ANESTHETIC ACTION PROGRESSIVELY DEVELOPS IN A NERVE, THE THRESHOLD FOR ELECTRICAL EXCITABILITY GRADUALLY INCREASES, THE RATE OF RISE OF THE ACTION POTENTIAL DECLINES, IMPULSE CONDUCTION SLOWS, & THE SAFETY FACTOR FOR CONDUCTION DECREASES; THESE FACTORS DECREASE THE PROBABILITY OF PROPAGATION OF THE ACTION POTENTIAL, AND NERVE CONDUCTION FAILS. /LOCAL ANESTHETICS/ POSTSYNAPTIC ACTION ... END-PLATE CURRENT IS MUCH PROLONGED BY PROCAINE. SIMILARLY, WHEN ... ADDED TO FLUID PERFUSING GANGLION, PREGANGLIONIC STIMULATION FAILS TO ELICIT POSTGANGLIONIC DISCHARGES & GANGLION CELLS BECOME INSENSITIVE TO STIMULATION BY ACETYLCHOLINE. IN ADDITION TO BLOCKING CONDITIONS IN NERVE AXONS IN THE PERIPHERAL NERVOUS SYSTEM, LOCAL ANESTHETICS INTERFERE WITH THE FUNCTION OF ALL ORGANS IN WHICH CONDUCTION OR TRANSMISSION OF IMPULSES OCCURS. ... EFFECTS ON ... CNS, THE AUTONOMIC GANGLIA, THE NEUROMUSCULAR JUNCTION, & ALL FORMS OF MUSCLE. /LOCAL ANESTHETICS/ |
| 分子式 |
C₁₃H₂₀N₂O₂
|
|---|---|
| 分子量 |
236.31
|
| 精确质量 |
236.152
|
| CAS号 |
59-46-1
|
| 相关CAS号 |
Procaine hydrochloride;51-05-8
|
| PubChem CID |
4914
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
373.6±22.0 °C at 760 mmHg
|
| 熔点 |
61ºC
|
| 闪点 |
179.8±22.3 °C
|
| 蒸汽压 |
0.0±0.8 mmHg at 25°C
|
| 折射率 |
1.543
|
| LogP |
2.36
|
| tPSA |
55.56
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
17
|
| 分子复杂度/Complexity |
222
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
MFDFERRIHVXMIY-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C13H20N2O2/c1-3-15(4-2)9-10-17-13(16)11-5-7-12(14)8-6-11/h5-8H,3-4,9-10,14H2,1-2H3
|
| 化学名 |
2-(diethylamino)ethyl 4-aminobenzoate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 该产品在溶液状态不稳定,请现配现用。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~423.17 mM)
H2O : ~1 mg/mL (~4.23 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (10.58 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (10.58 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (10.58 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 2 mg/mL (8.46 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶 (<60°C). 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.2317 mL | 21.1586 mL | 42.3173 mL | |
| 5 mM | 0.8463 mL | 4.2317 mL | 8.4635 mL | |
| 10 mM | 0.4232 mL | 2.1159 mL | 4.2317 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。