| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
Nuclear retinoic acid receptor γ (RAR-γ)
|
|---|---|
| 体外研究 (In Vitro) |
Palovarotene是一种强效的RAR-γ激动剂,在进行性骨化性纤维发育不良(FOP)的遗传模型和战斗相关HO的动物模型中,对预防软骨生成和HO特别有效。Palovarotene治疗可抑制全身炎症反应,包括细胞因子IL-6(p = 0.01)、TNF-α(p = 0.001)和IFN-γ(p = 0.03)以及通过术后第7天细胞浸润减少76%的局部炎症反应(POD)-7(p = 0.03). Palovarotene在体外可使成骨结缔组织祖细胞(CTP-O)集落减少98%(p = 0.04)和体内(p = 0.01) [2].
|
| 体内研究 (In Vivo) |
Palovarotene 抑制创伤引起的异位骨发育并介导成骨和创伤后骨形成。 Palovarotene 用于监测创伤后术中和皮下异位骨化 (HO)。在试验的前 14 天,从第 1 天或第 5 天开始,以 1 mg/kg/天的剂量口服 palovarotene。在长达 84 天的时间内定期观察 H2O 量、伤口破裂和相关过程。与载体动物相比,每剂量 palovarotene 可使 H2O 显着降低 50% 至 60% [1]。从损伤第一天开始,一半的 Acvr1cR206H/+ 小鼠每天接受 palovarotene 治疗,持续 14 天,而另一半小鼠则接受载体作为对照。根据 14 天后的 mCT 和 3D 图像重建分析,在载体-acvr1cR206H/+ 突变的目标腿中发现了大的组织肿块。然而,通过骨体积/总体积形成测量,接受 palovarotene 治疗的伴侣的 HO 显着降低,改善了 80% 以上[2]。
|
| 细胞实验 |
Palovarotene对体外CTP-O增殖的影响[2]
来自两只幼稚供体大鼠(第二代)股四头肌的肌源性大鼠间充质干细胞(rMSCs)以1 × 在补充有10%FBS的正常生长培养基中的6孔板中的103个细胞/孔,100 U/ml青霉素和100 μg Fungizone(Lonza)24 h,温度为37°C,空气中的5%二氧化碳完全加湿。对于分化研究组,将正常生长培养基改为成骨培养基,并补充不同浓度的帕洛沃汀(25,50125 nM)或DMSO(125 nM),每3天更换一次培养基。7天后,用PBS冲洗粘附的细胞集落两次,用100%甲醇固定5 室温下至少一分钟,空气干燥,用结晶紫溶液染色5 分钟,然后用蒸馏水漂洗以去除残留的染料。通过对治疗组不知情的读取器(TAD)使用光学显微镜对具有大于25个细胞/菌落的菌落进行计数。 |
| 动物实验 |
Rat Model for Combat-Related HO[2].
Sixty rats were randomly assigned to one of two treatment groups, Palovarotene or vehicle control (5% DMSO in corn oil), with six time points per group (n = 5 rats/treatment group/time point). Twelve rats were used as naïve controls and not exposed to injury. The 60 rats in the treatment groups were subjected to blast overpressure via a pneumatically driven shock tube (120 ± 7 kPa), femur fracture, soft tissue crush injury, and amputation through the zone of injury. Postoperative pain was managed using sustained-release buprenorphine (1.2 mg/kg) subcutaneously on the day of surgery with repeat dosing after 72-h, if needed, as previously described. Rats received via oral gavage (100 μl) of either Palovarotene (1.0 mg/kg; Atomax Chemicals, Shenzhen, China) or vehicle control every other day for 14 days beginning on POD)-1. Palovarotene purity confirmed greater than 98%. Postoperatively, rats were routinely monitored for signs of pain, weight loss, or wound complications and wounds that exhibited signs of infection or dehiscence were irrigated, débrided, and closed. Early euthanasia was performed if rats demonstrated a failure to thrive, persistent infection, or wound dehiscence after a third débridement. At study endpoint, rats were euthanized with pentobarbital (Fatal Plus; 390 mg/kg intraperitoneally; Patterson Veterinary, Devens, MA). Two rats died on POD-1, one from the 7 day vehicle control group and one from the 7 day Palovarotene group leaving a total of four animals in each of these two groups. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following oral administration of 20mg once daily in healthy adult subjects, the median Tmax was 4.6 hours, the mean Cmax was 140 ng/mL, and the mean AUC(0-τ) was 942 ng*hr/mL. At steady-state, the mean trough concentration of palovarotene was 3.5 ng/mL. The administration of palovarotene with a high-fat, high-calorie meal resulted in an approximate 40% increase in AUC, an approximate 16% increase in Cmax, and a delay in Tmax by approximately 2 hours when compared to its administration under fasting conditions. Following the administration of a 1mg radiolabeled dose of palovarotene in healthy subjects, approximately 97.1% of the administered radioactivity was recovered in the feces, with only 3.2% recovered in the urine. The mean (SD) apparent volume of distribution (Vd/F) is 237 (± 90.1) L following the administration of a single 20 mg dose with food. The apparent total body clearance of palovarotene is approximately 19.9 L/h. Metabolism / Metabolites Palovarotene undergoes extensive metabolism by CYP3A4 and, to a lesser extent, CYP2C8 and CYP2C19. Five metabolites have been observed in plasma: M1 (6,7-dihydroxy), M2 (6-hydroxy), M3 (7-hydroxy), M4a (6-oxo), and M4b (7-oxo). Following oral administration of palovarotene, the parent drug and its four main metabolites (M2, M3, M4a, and M4b) account for approximately 40% of total plasma exposure. The metabolites of palovarotene are functionally inactive, with M3 and M4b carrying 1.7% and 4.2% of the activity of their parent compound, respectively. Biological Half-Life The mean elimination half-life of palovarotene at steady-state is 8.7 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
The protein binding of palovarotene is 97.9% to 99.6% in vitro. |
| 参考文献 |
[1]. Pavey GJ, et al. Targeted stimulation of retinoic acid receptor-γ mitigates the formation of heterotopic ossification in an established blast-related traumatic injury model. Bone. 2016 Sep;90:159-67.
[2]. Chakkalakal SA, et al. Palovarotene Inhibits Heterotopic Ossification and Maintains Limb Mobility and Growth in Mice With the Human ACVR1(R206H) Fibrodysplasia Ossificans Progressiva (FOP) Mutation. J Bone Miner Res. 2016 Sep;31(9):1666-75. [3]. Lees-Shepard JB, et al. Palovarotene reduces heterotopic ossification in juvenile FOP mice but exhibits pronounced skeletal toxicity. Elife. 2018 Sep 18;7. pii: e40814. [2]. Palovarotene inhibits connective tissue progenitor cell proliferation in a rat model of combat-related heterotopic ossification. J Orthop Res. 2018 Apr;36(4):1135-1144. |
| 其他信息 |
Pharmacodynamics
Palovarotene exerts its pharmacologic effects by inhibiting the pathway(s) responsible for heterotopic ossification in patients with FOP. It is orally bioavailable and can be administered once daily, with allowances for short-term increases in dosage in the event of a flare-up. As with other retinoids, palovarotene can cause birth defects, and it should not be used by patients who are, or intend to become, pregnant. Palovarotene is contraindicated in patients of childbearing potential unless a number of pregnancy prevention strategies are met (e.g. effective contraception, regular pregnancy testing). Palovarotene may also cause a premature physeal closure in growing children. Physeal growth plates should be monitored every 3 months throughout therapy, or more frequently if evidence of adverse effects on growth are observed. At doses up to 2.5 times the maximum recommended dose, palovarotene does not prolong the QT interval to any clinically relevant extent. Palovarotene binds to retinoic acid receptor gamma (RARγ) with a 10-fold greater affinity compared to retinoic acid receptor alpha or beta. In animal models of Fibrodysplasia Ossificans Progressiva (BMP implant in WT mouse, Q207D mouse model, R206H mouse model), palovarotene decreased heterotopic ossification (HO) in a dose-dependent manner as well as inflammatory and fibroproliferative responses at the sites of injury. Additionally, palovarotene also outperformed corticosteroids in preventing HO, with a dexamethasone treatment of 4.4 mg/kg/day for 4 days demonstrating no clinical efficacy on heterotropic bone volume. |
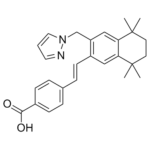
| 分子式 |
C27H30N2O2
|
|---|---|
| 分子量 |
414.54
|
| 精确质量 |
414.23
|
| 元素分析 |
C, 78.23; H, 7.29; N, 6.76; O, 7.72
|
| CAS号 |
410528-02-8
|
| PubChem CID |
10295295
|
| 外观&性状 |
Off-white to yellow solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
592.3±50.0 °C at 760 mmHg
|
| 闪点 |
312.0±30.1 °C
|
| 蒸汽压 |
0.0±1.8 mmHg at 25°C
|
| 折射率 |
1.595
|
| LogP |
7.63
|
| tPSA |
55.12
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
31
|
| 分子复杂度/Complexity |
662
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C(O)C1=CC=C(/C=C/C2=C(CN3N=CC=C3)C=C4C(C)(C)CCC(C)(C)C4=C2)C=C1
|
| InChi Key |
YTFHCXIPDIHOIA-DHZHZOJOSA-N
|
| InChi Code |
InChI=1S/C27H30N2O2/c1-26(2)12-13-27(3,4)24-17-22(18-29-15-5-14-28-29)21(16-23(24)26)11-8-19-6-9-20(10-7-19)25(30)31/h5-11,14-17H,12-13,18H2,1-4H3,(H,30,31)/b11-8+
|
| 化学名 |
4-((1E)-2-(5,5,8,8-Tetramethyl-3-(1H-pyrazol-1-ylmethyl)-5,6,7,8-tetrahydronaphthalen-2-yl)ethenyl)benzoic acid
|
| 别名 |
RO-3300074; R-667; RO 3300074; R 667; RO3300074; R667; Sohonos
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~25 mg/mL (~60.31 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.03 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (5.02 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4123 mL | 12.0616 mL | 24.1231 mL | |
| 5 mM | 0.4825 mL | 2.4123 mL | 4.8246 mL | |
| 10 mM | 0.2412 mL | 1.2062 mL | 2.4123 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
 Injury-induced heterotopic ossification inAcvr1cR206H/+mutant mice is inhibited by the RARγ agonist Palovarotene. |
|---|
 Palovarotene preserves long bone growth and growth plate organization inPrrx1-R206Hmice.J Bone Miner Res.2016 Sep;31(9):1666-75. |
 Chondrocyte proliferation and progression are altered inPrrx1-R206Hgrowth plates.J Bone Miner Res.2016 Sep;31(9):1666-75. |