
| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
DYRK1A (IC50 = 2000 nM); MAPK (IC50 = 8000 nM); Cdk4/cyclin D3 (IC50 = 9 nM); Cdk4/cyclin D1 (IC50 = 11 nM); Cdk6/cyclin D2 (IC50 = 16 nM)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:PD 0332991 对其他蛋白激酶包括 EGFR、FGFR、PGFR、IR 几乎没有影响。 PD 0332991 是 Cdk4 的非 ATP 竞争性抑制剂。 PD 0332991 抑制 MDA-MB-435 乳腺癌细胞,IC50 为 66 nM,这是由于 Ser780 处的 Rb 磷酸化减少所致。 PD 0332991 抑制胸苷掺入 Rb 阳性人乳腺癌、结肠癌、肺癌以及人白血病的 DNA,IC50 值范围为 0.04-0.17 μM。 PD 0332991 在 Rb 阴性细胞中没有显示出活性。 PD 0332991 导致 MDA-MB-453 乳腺癌细胞和 Colo-205 癌细胞中 G1 期细胞积聚。 PD 0332991 还在 5T33MM 骨髓瘤细胞(免疫活性模型)中显示出活性,并使细胞对硼替佐米的杀伤敏感。 PD 0332991 抑制 luminal ER 阳性以及 HER2 扩增的乳腺癌细胞系,包括 MDA-MB-175、ZR-75-30、CAMA-1、MDA-MB-134、HCC-202 和 UACC-893。 PD 0332991 增强这些细胞系中他莫昔芬和曲妥珠单抗的活性。 PD 0332991 增强 MCF7 他莫昔芬耐药细胞中他莫昔芬的敏感性。最近的一项研究表明,PD 0332991 可以抑制恶性横纹肌瘤 (MRT) 细胞系,包括 MP-MRT-AN、KP-MRT-RY、G401、KP-MRT-NS,且 MRT 细胞系对 PD 0332991 的敏感性呈反比。与p16的表达相关。激酶测定:在 DMSO 中制备 PD0332991 的储备溶液。 CDK 测定在 96 孔过滤板中进行。所有CDK-细胞周期蛋白激酶复合物均通过杆状病毒感染在昆虫细胞中表达并纯化。检测的底物是与 GST 融合的 pRb 片段(氨基酸 792-928)(GST·RB-Cterm)。每孔总体积为 0.1 mL,含有终浓度 20 mM Tris-HCl、pH 7.4、50 mM NaCl、1 mM 二硫苏糖醇、10 mM MgCl2、25 μM ATP(对于 CDK4-细胞周期蛋白 D1、CDK6-细胞周期蛋白 D2、和 CDK6-细胞周期蛋白 D3)或 12 μM ATP(对于 CDK2-细胞周期蛋白 E、CDK2-细胞周期蛋白 A 和 CDC2-细胞周期蛋白 B),含 0.25 μCi [γ-32P]ATP、20 ng 酶、1 μg GST·RB -Cterm 和 PD 0332991 (0.001-0.1μM)。除 [γ-32P]ATP 外的所有组分均添加至孔中,并将板置于板混合器上 2 分钟。通过添加 [γ-32P]ATP 开始反应,并将板在 25 °C 下孵育 15 分钟。通过添加 0.1 mL 20% 三氯乙酸终止反应,并将板在 4 °C 下保持至少 1 小时,以使底物沉淀。然后用 0.2 mL 10% 三氯乙酸洗涤孔 5 次,并用 β 板计数器测定放射性掺入。细胞测定:细胞(肿瘤细胞系包括 MDA-MB-435、ZR-75-1、T-47D、MCF-7、H1299、Colo-205、MDA-MB-468、H2009、CRRF-CEM 和 K562)以每孔 2 × 104 的密度接种到 96 孔板中并孵育过夜。将 PD 0332991 (0.01-1 μM) 添加到孔中,并在 37 °C 下再孵育 24 小时。将[14C]胸苷(0.1μCi)添加到每个孔中,并允许放射性标记的掺入持续72小时。用β板计数器测定掺入的放射性。
|
||
| 体内研究 (In Vivo) |
PD 0332991 表明 150 mg/kg 的 MDA-MB-435 异种移植物中肿瘤完全停滞。 PD 0332991 还通过消除肿瘤组织中的磷酸化 Rb 和增殖标记物 Ki-67 以及在 E2F 转录控制下下调基因,在多个人类肿瘤异种移植物中显示出广谱抗肿瘤活性。
|
||
| 酶活实验 |
Palbociclib是一种CDK4/6抑制剂,被批准用于转移性雌激素受体阳性乳腺癌。除了G1细胞周期阻滞外,palbociclib治疗还会导致细胞衰老,这是一种不易用CDK4/6抑制来解释的表型。为了确定palbociclib诱导衰老的分子机制,我们对MCF7乳腺癌细胞进行了热蛋白质组分析。除了影响已知的CDK4/6靶标外,palbociclib诱导20S蛋白酶体的热稳定,尽管它没有直接结合。我们进一步表明,帕博西尼治疗增加蛋白酶体活性独立于泛素途径。这导致细胞衰老,这可以通过蛋白酶体抑制剂抵消。palbociclib诱导的蛋白酶体激活和衰老是由ECM29蛋白酶体关联降低介导的。ECM29的缺失激活蛋白酶体,阻断细胞增殖,并诱导衰老样表型。最后,我们发现ECM29 mRNA水平可预测接受内分泌治疗的乳腺癌患者的无复发生存。总之,热蛋白质组分析鉴定了蛋白酶体和ECM29蛋白是乳腺癌细胞中palbociclib活性的介质[2]。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Palbociclib presents a linear pharmacokinetic profile and its peak plasma concentration was observed 6-12 hours after oral administration. The oral bioavailability is reported to be of 46% with a steady-state reached after 8 days and a median accumulation ratio of 2.4. The absorption of palbociclib is significantly reduced under fasting conditions and hence, food intake is recommended when this drug is administered. The main route of elimination of palbociclib is through feces after hepatic metabolism while renal clearance seems to play a minor role accounting only for 17.5% of the eliminated dose. The mean apparent distribution of palbociclib is 2583 L which suggests that palbociclib penetrates extensively into peripheral tissues. The mean apparent oral clearance of palbociclib is of 63.1 L/h. Metabolism / Metabolites Palbociclib is mainly hepatically transformed. the metabolism is mainly performed by the activities of the cytochrome P450 isoenzyme 3A and the sulfotransferase 2A1. The metabolism of palbociclib is represented mainly by reactions of oxidation and sulfonation followed by acylation and glucuronidation as minor reactions. After its metabolism, palbociclib forms mainly inactive glucuronide and sulfamic acid conjugates. The major circulating metabolite, accounting for 1.5% of the dose in excreta is is the glucuronide conjugate. Biological Half-Life The mean plasma elimination half-life of palbociclib is 29 hours. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In the large clinical trials, adverse events were common and led to dose reductions in one-third of patients and discontinuation in 8%. Publications on the efficacy and safety of palbociclib rarely mentioned serum ALT elevations or hepatotoxicity. In a study of women with refractory, metastatic breast cancer, serum ALT elevations occurred in 6% [2% over 5 times ULN] receiving palbociclib and fulvestrant compared to 3% [none over 5 times ULN] on fulvestrant alone. Since its approval and more widescale use, there have been several reports of prominent ALT elevations arising after 2 or 3 cycles of palbociclib, that improved on discontinuation and recurred rapidly when restarted. Serum bilirubin and alkaline phosphatase levels were normal and symptoms were not mentioned. In addition, there have been rare reports of patients with refractory metastatic breast cancer who developed pseudocirrhosis within 2 to 3 months of starting palbociclib presenting with fatigue, jaundice and ascites with only modest elevations in serum aminotransferase and alkaline phosphatase levels. Imaging revealed a severely nodular liver, but liver histology showed desmoplastic changes in areas of necrotic metastatic tumor without cirrhosis. The liver also had vascular changes suggestive of sinusoidal obstruction syndrome, changes possibly caused by the dramatic involution of the metastatic tumor tissue combined with vascular damage. Pseudocirrhosis has been reported with other highly successful antineoplastic therapies of cancer metastatic to the liver, but the frequency is rare. Likelihood score: C (probable rare cause of clinically apparent liver injury that may represent pseudocirrhosis from nodular transformation of the liver in response to necrosis of hepatic metastases). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of palbociclib during breastfeeding. Because palbociclib is 85% bound to plasma proteins, the amount in milk is likely to be low. However, its half-life is about 29 hours and it might accumulate in the infant. It is also given in combination with letrozole or fulvestrant, which may increase the risk to the infant. The manufacturer recommends that breastfeeding be discontinued during palbociclib therapy and for 3 weeks after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Binding of palbociclib to human plasma proteins in vitro accounts for approximately 85% of the administered dose. |
||
| 参考文献 |
[1]. Mol Cancer Ther.2004 Nov;3(11):1427-38;
Cell Death Dis.2018 Apr 18;9(5):446; EMBO J.2018 Apr 18. pii: e98359. doi: 10.15252/embj.201798359. |
||
| 其他信息 |
Palbociclib is a member of the class of pyridopyrimidines that is 2-{[5-(piperazin-1-yl)pyridin-2-yl]amino}pyrido[2,3-d]pyrimidin-7-one bearing additional methyl, acetyl and cyclopentyl substituents at positions 5, 6 and 8 respectively. It is used in combination with letrozole for the treatment of metastatic breast cancer. It has a role as an EC 2.7.11.22 (cyclin-dependent kinase) inhibitor and an antineoplastic agent. It is a pyridopyrimidine, an aminopyridine, a secondary amino compound, a member of piperidines, an aromatic ketone, a member of cyclopentanes and a tertiary amino compound.
Palbociclib is a piperazine pyridopyrimidine that acts in the cell cycle machinery. It is a second generation cyclin-dependent kinase inhibitor selected from a group of pyridopyrimidine compounds due to its favorable physical and pharmaceutical properties. Palbociclib was developed by Pfizer Inc after the discovery that identified the cyclin-dependent kinases as key regulators of cell growth. It was originally FDA approved on March 2015 for the treatment of HR-positive, HER2-negative advanced or metastatic breast cancer and its indications were updated in April 2019 to include male patients based on findings from postmarketing reports and electronic health records demonstrating safety and clinical efficacy. Palbociclib is a Kinase Inhibitor. The mechanism of action of palbociclib is as a Kinase Inhibitor, and Cytochrome P450 3A Inhibitor. Palbociclib is a unique cyclin-dependent kinase inhibitor that is used in combination with aromatase inhibitors in the treatment of postmenopausal women with metastatic breast cancer. Palbociclib is associated with transient and usually mild elevations in serum aminotransferase during therapy and to an unusual form of liver injury called pseudocirrhosis caused by shrinkage of tumor metastases in the liver combined with desmoplastic changes and vascular damage, that can be severe, progressive and even fatal. Palbociclib is an orally available cyclin-dependent kinase (CDK) inhibitor with potential antineoplastic activity. Palbociclib selectively inhibits cyclin-dependent kinase 4 (CDK4) and 6 (CDK6), thereby inhibiting retinoblastoma (Rb) protein phosphorylation early in the G1 phase leading to cell cycle arrest. This suppresses DNA replication and decreases tumor cell proliferation. CDK4 and 6 are serine/threonine kinases that are upregulated in many tumor cell types and play a key role in the regulation of cell cycle progression. See also: Palbociclib Isethionate (is active moiety of). Drug Indication Palbociclib is indicated in combination with [letrozole] as initial endocrine-based therapy for the treatment of human epidermal growth factor receptor type 2 (HER2)-negative and hormone receptor(HR)-positive tumors in adult patients with advanced/metastatic breast cancer. It is as well approved in combination with [fulvestrant] in patients with disease progression with prior endocrine therapy. In the official labeling, the use of palbociclib should be accompanied with either an aromatase inhibition, no restricted to letrozole, as initial endocrine-based therapy in postmenopausal women or in man. The breast cancer starts as a group of cancer cells that grow into and destroy the nearby breast tissue. This growth can spread into other parts of the body which is called metastasis. According to the location of the cancer cells, it can be categorized in ductal carcinoma and lobular carcinoma. However, other types of breast cancer include inflammatory breast cancer, Paget disease of the breast, triple negative breast cancer non-Hodgkin lymphoma and soft tissue sarcoma. In males, breast cancer is usually treated as the cases of postmenopausal women and almost all the cases are ductal carcinoma. FDA Label Ibrance is indicated for the treatment of hormone receptor (HR) positive, human epidermal growth factor receptor 2 (HER2) negative locally advanced or metastatic breast cancer : in combination with an aromatase inhibitor; in combination with fulvestrant in women who have received prior endocrine therapy. In pre- or perimenopausal women, the endocrine therapy should be combined with a luteinizing hormone releasing hormone (LHRH) agonist. Treatment of Ewing sarcoma Treatment of breast malignant neoplasms Mechanism of Action Palbociclib is a cyclin-dependent kinase 4/6 (CDK4/6) inhibitor that acts by binding to the ATP pocket with an IC50 in the range of 9-15 nmol/L. It is important to consider that it presents low to absent activity against other kinases. The CDK4/6 kinase is involved, with coregulatory partner cyclin D, in the G1-S transition. Hence, inhibition of this step prevents cell cycle progression in cells in whose this pathway is functioning. This step includes the pathways of the phosphorylation of retinoblastoma protein and the E2F family of transcription factors. Synovial sarcoma is a highly aggressive but rare form of soft tissue malignancy that primarily affects the extremities of the arms or legs, for which current chemotherapeutic agents have not been proven to be very effective. The cyclin-dependent kinase 4/6-retinoblastoma protein (CDK4/6-Rb) pathway of cell cycle control is known to be aberrant in a large proportion of cancers. Recently, CDK4 inhibitors have successfully been used pre-clinically for the treatment of many human cancers, and in 2015, following the success of clinical trials, the FDA approved the first selective CDK4/6 inhibitor, palbociclib, for the treatment of endocrine therapy resistant breast cancers. However, the expression and therapeutic potential of targeting CDK4 in synovial sarcoma remains unclear. In the present study, we report that CDK4 is highly expressed in human synovial sarcoma, and high CDK4 expressions are associated with poor prognosis in sarcomas patients and the clinical stage and the TNM grade in synovial sarcoma patients. Knockdown of CDK4 with specific small interference RNAs inhibits cell proliferation and enhances apoptotic effects in synovial sarcoma cells. CDK4 inhibitor palbociclib suppresses synovial sarcoma cell proliferation and growth in a dose and time-dependent manner. Palbociclib also inhibits the CDK4/6-Rb signaling pathway and promotes cell apoptosis without changing CDK4/6 protein levels, suggesting that palbociclib only represses the hyper-activation, not the expression of CDK4/6. Flow cytometry analysis reveals that palbociclib induces G1 cell-cycle arrest and apoptotic effects by targeting the CDK4/6-Rb pathway in synovial sarcoma cells. Furthermore, wound healing assays demonstrate that inhibition of the CDK4/6-Rb pathway by palbociclib significantly decreases synovial sarcoma cell migration in vitro. Our study highlights the importance of the CDK4/6-Rb pathway in human synovial sarcoma pathogenesis, and the role of the current selective CDK4/6 inhibitor, palbociclib, as a potential promising targeted therapeutic agent in the treatment of human synovial sarcoma.[1] |
| 精确质量 |
603.2553798
|
|---|---|
| CAS号 |
2757498-64-7
|
| 相关CAS号 |
571190-30-2; 827022-33-3 (isethionate salt); 827022-32-2 (HCl); 1628752-83-9 (Palbociclib D8); 2366269-23-8 (Palbociclib-propargyl) |
| PubChem CID |
164887438
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| tPSA |
199Ų
|
| InChi Key |
HOLXHPZTHUPIFD-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C24H29N7O2.C5H4N2O4/c1-15-19-14-27-24(28-20-8-7-18(13-26-20)30-11-9-25-10-12-30)29-22(19)31(17-5-3-4-6-17)23(33)21(15)16(2)32;8-3-1-2(4(9)10)6-5(11)7-3/h7-8,13-14,17,25H,3-6,9-12H2,1-2H3,(H,26,27,28,29);1H,(H,9,10)(H2,6,7,8,11)
|
| 化学名 |
6-acetyl-8-cyclopentyl-5-methyl-2-[(5-piperazin-1-ylpyridin-2-yl)amino]pyrido[2,3-d]pyrimidin-7-one;2,4-dioxo-1H-pyrimidine-6-carboxylic acid
|
| 别名 |
Palbociclib orotate; Palbociclib (orotate);
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Evaluation of IC50concentrations of the CDK inhibitors dinaciclib and palbociclib on proliferation, and their effects on CDK-Rb-E2F signaling in human HPASMCs from healthy donors and IPAH patients.Nat Commun.2019May 17;10(1):2204. |
|---|
Effects of the CDK inhibitors dinaciclib and palbociclib on proliferation, cell cycle, and apoptosis.Nat Commun.2019May 17;10(1):2204. |
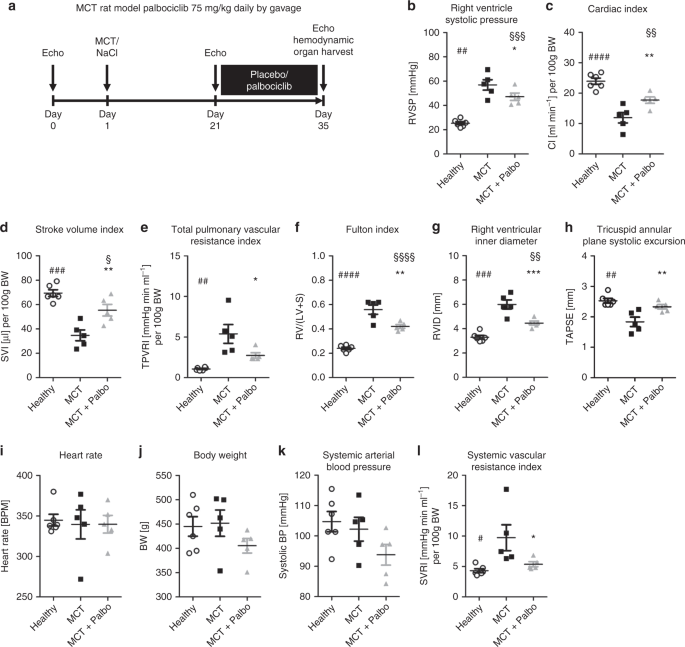 Effects of palbociclib on disease progression in the MCT rat model of pulmonary arterial hypertension.Nat Commun.2019May 17;10(1):2204. |
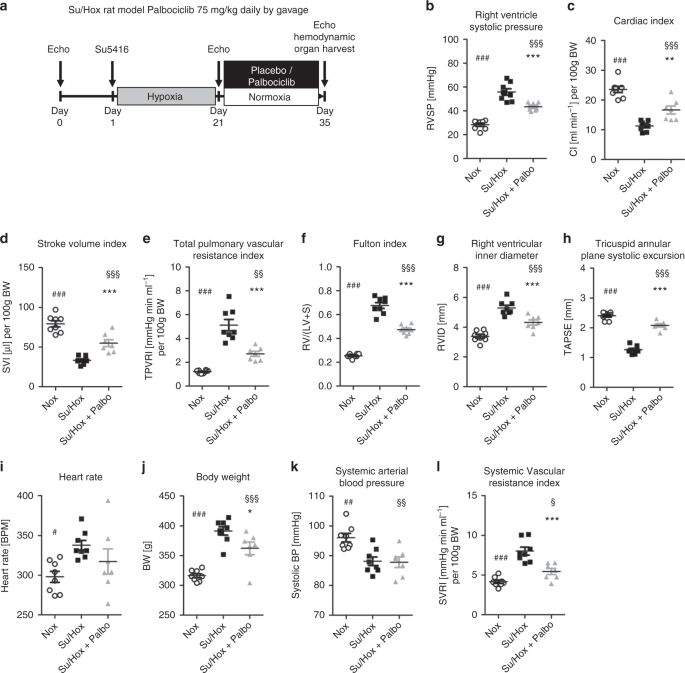 Effects of palbociclib on disease progression in the Su/Hox rat model of pulmonary arterial hypertension.Nat Commun.2019May 17;10(1):2204. |
|---|
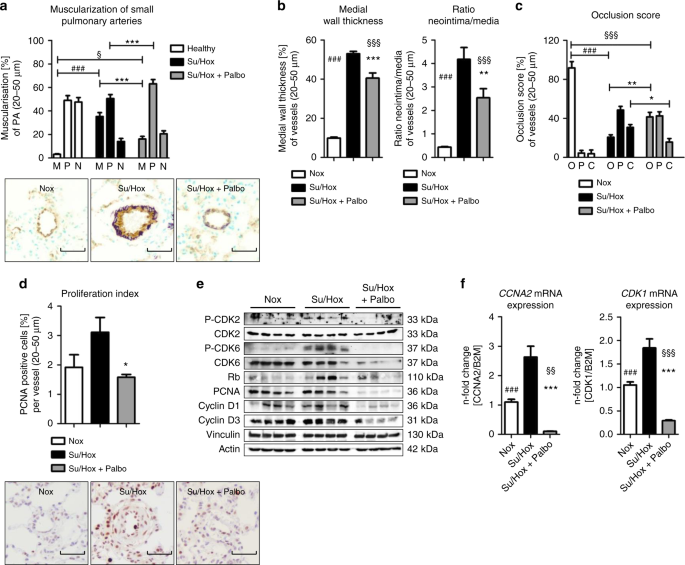 Ex vivo analyses of lung tissue for reversal of remodeling and in vivo drug efficacy in the Su/Hox model.Nat Commun.2019May 17;10(1):2204. |
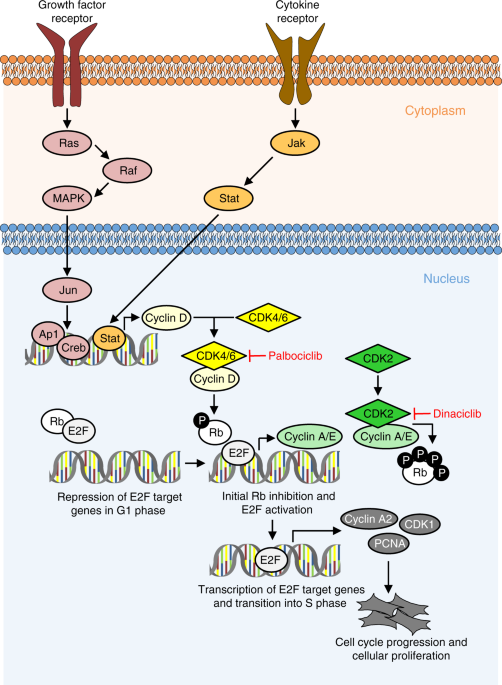 Proposed mechanism of action of palbociclib and dinaciclib in PAH. Multiple growth factors, cytokines, and mitogens induce the activation of cyclin-dependent kinases (CDKs), e.g., by increasing the expression of cyclin D1.Nat Commun.2019May 17;10(1):2204. |