| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
Tubulin
|
|---|---|
| 体外研究 (In Vitro) |
尽管卡铂和紫杉醇的标准初级治疗方案的初始反应率为80%,但大多数癌症卵巢患者将在五年内出现不治之症复发,并将接受多种连续姑息治疗。因此,对化疗药物的需求很大,化疗药物不仅在临床上有效,而且具有可接受的副作用。紫杉醇多颖壳(PPX)是一种最近开发的紫杉烷,其中紫杉醇与聚(l-谷氨酸)结合,使其水溶性,减少超敏反应,并优先将其靶向肿瘤[1]。
|
| 体内研究 (In Vivo) |
紫杉烷仍然是延长去势抵抗转移性前列腺癌症生存期的唯一药物,但它们对该疾病自然史的影响不大。我们试图检验这样一种假设,即通过使用雌二醇调节的紫杉醇大分子聚合物药物偶联物来增加紫杉烷化疗对肿瘤的输送,可以扩大这类药物的效用。尽管接受了标准激素治疗,但在含多西他赛的化疗后仍进展的前列腺转移性腺癌患者接受了为期4周的经皮雌二醇(0.2 mg/24小时)治疗,随后每28天接受一次相同剂量的经皮雌激素和紫杉醇聚晶(PPX;150 mg/m静脉注射)治疗。主要目的是确定使用一部分血清前列腺特异性抗原(PSA)下降50%或更多的患者测量的方案活性水平。一项旨在确定反应率≥25%的两阶段II期研究要求在第一阶段的21名患者中有3名反应者。在2007年3月至2008年5月期间,21名接受了两种早期化疗方案中位数的患者参加了这项试验。在仅使用雌二醇的治疗阶段,没有患者的PSA下降超过50%,五名患者的PSA降幅较小,范围在8.8%至34.1%之间。在添加PPX后,没有患者的PSA下降≥50%,可测量的疾病也没有反应。进展的中位时间为4周。总之,这种低剂量透皮雌二醇诱导和PPX方案在紫杉烷预处理的去势耐受性前列腺癌症患者中没有活性[2]。
|
| 动物实验 |
Taxanes remain the only agents to extend survival in castration-resistant metastatic prostate cancer, but their impact on the natural history of this disease is modest. We sought to test the hypothesis that increased delivery of taxane chemotherapy to the tumor through the use of a macromolecular polymer-drug conjugate of paclitaxel modulated by estradiol could extend the utility of this class of drugs. Patients with metastatic adenocarcinoma of the prostate who progressed despite standard hormonal therapy and after docetaxel-containing chemotherapy were treated with transdermal estradiol (0.2 mg/24 h) for 4 weeks followed by the same dose of transdermal estradiol and paclitaxel poliglumex (PPX; 150 mg/m intravenous) every 28 days. The primary objective was to determine the level of activity of the regimen measured using a fraction of patients who experienced a confirmed decline in serum prostate-specific antigen (PSA) of 50% or more. A two-stage phase II study designed to identify a response rate of > or =25% required three responders among 21 patients in the first stage. Twenty-one patients who received a median of two earlier chemotherapy regimens were enrolled in the trial between March 2007 and May 2008. During the estradiol-only treatment phase, no patient had a PSA decline in excess of 50% and lesser PSA declines that ranged from 8.8 to 34.1% were seen in five patients. No patients achieved a > or =50% PSA decline following the addition of PPX and there were no responses in measurable disease. The median time to progression was 4 weeks. In conclusion, this regimen of low-dose transdermal estradiol induction followed by PPX does not have activity in taxane pretreated patients with castration-resistant prostate cancer.[2]
|
| 参考文献 |
[1]. Paclitaxel poliglumex for ovarian cancer. Expert Opin Investig Drugs. 2011 Jun;20(6):813-21.
[2]. A phase II study of paclitaxel poliglumex in combination with transdermal estradiol for the treatment of metastatic castration-resistant prostate cancer after docetaxel chemotherapy. Anticancer Drugs. 2010 Apr;21(4):433-8. |
| 其他信息 |
Paclitaxel Poliglumex is the agent paclitaxel linked to a biodegradable, water-soluble polyglutamate polymer with antineoplastic properties. The polyglutamate residue increases the water solubility of paclitaxel and allows delivery of higher doses than those achievable with paclitaxel alone. Paclitaxel promotes microtubule assembly and prevents microtubule depolymerization, thus interfering with normal mitosis.
Despite an 80% initial response rate to the standard primary regimen of carboplatin and paclitaxel, most women with ovarian cancer will experience recurrence with incurable disease within five years and will be treated with several successive palliative regimens. Consequently, a significant need exists for chemotherapeutic agents, which are not only clinically efficacious, but have acceptable side-effect profiles. Paclitaxel poliglumex (PPX) is a recently developed taxane in which paclitaxel is conjugated to poly(l-glutamic acid), which renders it water soluble, reduces hypersensitivity reactions and preferentially targets it to the tumor. Areas covered: This review covers pre-clinical pharmacokinetic data and key Phase I and II clinical trial results in ovarian cancer. Expert opinion: While PPX is active in ovarian cancer, it is unclear at present whether it offers significant benefit in terms of its side-effect profile or outcomes over a standard taxane-based regimen as first-line therapy, or what role it will have in maintenance therapy as studies are ongoing.[1] |
| 分子式 |
C52H60N2O18
|
|---|---|
| 分子量 |
1001.04
|
| 精确质量 |
853.33
|
| 元素分析 |
C, 62.39; H, 6.04; N, 2.80; O, 28.77
|
| CAS号 |
263351-82-2
|
| 相关CAS号 |
186040-50-6 (ceribate); 117527-50-1 (Paclitaxel-Succinic acid); 33069-62-4; 263351-82-2 (Poliglumex)
|
| PubChem CID |
134715513
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
957.1±65.0 °C at 760 mmHg
|
| 闪点 |
532.6±34.3 °C
|
| 蒸汽压 |
0.0±0.3 mmHg at 25°C
|
| 折射率 |
1.637
|
| LogP |
7.38
|
| tPSA |
322Ų
|
| 氢键供体(HBD)数目 |
7
|
| 氢键受体(HBA)数目 |
19
|
| 可旋转键数目(RBC) |
18
|
| 重原子数目 |
72
|
| 分子复杂度/Complexity |
1940
|
| 定义原子立体中心数目 |
12
|
| SMILES |
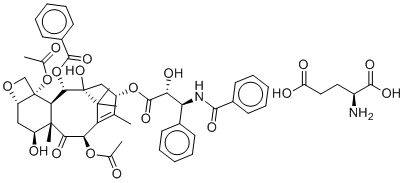
O1C[C@@]2([C@H]1C[C@@H]([C@@]1(C)C([C@@H](C3=C(C)[C@H](C[C@](C3(C)C)([C@H]([C@H]21)OC(C1C=CC=CC=1)=O)O)OC([C@@H]([C@H](C1C=CC=CC=1)NC(C1C=CC=CC=1)=O)O)=O)OC(C)=O)=O)O)OC(C)=O
|
| InChi Key |
ZPUHVPYXSITYDI-ZYOSQDPASA-N
|
| InChi Code |
InChI=1S/C47H51NO14.C5H9NO4/c1-25-31(60-43(56)36(52)35(28-16-10-7-11-17-28)48-41(54)29-18-12-8-13-19-29)23-47(57)40(61-42(55)30-20-14-9-15-21-30)38-45(6,32(51)22-33-46(38,24-58-33)62-27(3)50)39(53)37(59-26(2)49)34(25)44(47,4)56-3(5(9)10)1-2-4(7)8/h7-21,31-33,35-38,40,51-52,57H,22-24H2,1-6H3,(H,48,54)3H,1-2,6H2,(H,7,8)(H,9,10)/t31-,32-,33-,35-,36+,37+,38-,40-,45+,46-,47-3-/m00/s1
|
| 化学名 |
(2aS,4S,4aS,6R,9S,11R,12S,12aR,12bS)-9-(((2R,3S)-3-benzamido-2-hydroxy-3-phenylpropanoyl)oxy)-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxete-6,12b(2aH)-diyl
diacetate L-glutamate
|
| 别名 |
PPX PG-TXL; Paclitaxel Poliglumex; CT-2103; CT2103; Paclitaxel Poliglumex; 263351-82-2; 2-aminopentanedioic acid;[(1R,4S,10S)-4,12-diacetyloxy-15-(3-benzamido-2-hydroxy-3-phenylpropanoyl)oxy-1,9-dihydroxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.03,10.04,7]heptadec-13-en-2-yl] benzoate; PG-TXL; CT 2103; PPX; Opaxio; Xyotax
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.9990 mL | 4.9948 mL | 9.9896 mL | |
| 5 mM | 0.1998 mL | 0.9990 mL | 1.9979 mL | |
| 10 mM | 0.0999 mL | 0.4995 mL | 0.9990 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。