| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| 50g |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
奥西普嗪以剂量依赖性方式诱导细胞凋亡。当 caspase-3 参与时,奥沙普秦会增强其活性;然而,当它静止时,它就不会。 50 μM 奥沙普秦可抑制 NF-κB 的激活。当试剂 IκBα 激活 IKK 系统时,奥沙普秦会阻止其发生[1]。奥沙普秦 (oxaprozin) (100 μM) 诱导的促凋亡作用最高,它也极大地促进 CD40L 处理的单核细胞的凋亡。使用奥西普嗪治疗可防止 CD40L 触发的 Akt 和 NF-κB (p65) 磷酸化 [2]。
|
||
|---|---|---|---|
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Oxaprozin is 95% absorbed after oral administration. Food may reduce the rate of absorption of oxaprozin, but the extent of absorption is unchanged. Antacids do not significantly affect the extent and rate of oxaprozin absorption. Oxaprozin is expected to be excreted in human milk based on its physical-chemical properties; however, the amount of oxaprozin excreted in breast milk has not been evaluated. Approximately 95% of oxaprozin is metabolized by the liver. Approximately 5% of the oxaprozin dose is excreted unchanged in the urine. Sixty-five percent (65%) of the dose is excreted in the urine and 35% in the feces as metabolite. Biliary excretion of unchanged oxaprozin is a minor pathway. Several oxaprozin metabolites have been identified in human urine or feces. 11 to 17 L/70 kg In dose proportionality studies utilizing 600, 1200 and 1800 mg doses, the pharmacokinetics of oxaprozin in healthy subjects demonstrated nonlinear kinetics of both the total and unbound drug in opposite directions, i.e., dose exposure related increase in the clearance of total drug and decrease in the clearance of the unbound drug. Decreased clearance of the unbound drug was related predominantly to a decrease in the volume of distribution and not an increase in the half-life. This phenomenon is considered to have minimal impact on drug accumulation upon multiple dosing. The apparent volume of distribution (Vd/F) of total oxaprozin is approximately 11-17 L/70 kg. Oxaprozin is 99% bound to plasma proteins, primarily to albumin. At therapeutic drug concentrations, the plasma protein binding of oxaprozin is saturable, resulting in a higher proportion of the free drug as the total drug concentration is increased. With increases in single doses or following repetitive once-daily dosing, the apparent volume of distribution and clearance of total drug increased, while that of unbound drug decreased due to the effects of nonlinear protein binding. Oxaprozin penetrates into synovial tissues of rheumatoid arthritis patients with oxaprozin concentrations 2-fold and 3-fold greater than in plasma and synovial fluid, respectively. Oxaprozin is expected to be excreted in human milk based on its physical-chemical properties; however, the amount of oxaprozin excreted in breast milk has not been evaluated. Daypro is 95% absorbed after oral administration. Food may reduce the rate of absorption of oxaprozin, but the extent of absorption is unchanged. Antacids do not significantly affect the extent and rate of Daypro absorption. It is not known whether oxaprozin is distributed into human breast milk. However, it is distributed into the milk of lactating rats. Approximately 5% of the oxaprozin dose is excreted unchanged in the urine. Sixty-five percent (65%) of the dose is excreted in the urine and 35% in the feces as metabolite. Biliary excretion of unchanged oxaprozin is a minor pathway, and enterohepatic recycling of oxaprozin is insignificant. Upon chronic dosing the accumulation half-life is approximately 22 hours. The elimination half-life is approximately twice the accumulation half-life due to increased binding and decreased clearance at lower concentrations. For more Absorption, Distribution and Excretion (Complete) data for OXAPROZIN (8 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic. Ester and ether glucuronide are the major conjugated metabolites of oxaprozin, and do not have significant pharmacologic activity. Several oxaprozin metabolites have been identified in human urine or feces. Oxaprozin is primarily metabolized by the liver, by both microsomal oxidation (65%) and glucuronic acid conjugation (35%). Ester and ether glucuronide are the major conjugated metabolites of oxaprozin. On chronic dosing, metabolites do not accumulate in the plasma of patients with normal renal function. Concentrations of the metabolites in plasma are very low. Oxaprozin's metabolites do not have significant pharmacologic activity. The major ester and ether glucuronide conjugated metabolites have been evaluated along with oxaprozin in receptor binding studies and in vivo animal models and have demonstrated no activity. A small amount (<5%) of active phenolic metabolites are produced, but the contribution to overall activity is limited. Biological Half-Life 54.9 hours Upon chronic dosing the accumulation half-life is approximately 22 hours. The elimination half-life is approximately twice the accumulation half-life due to increased binding and decreased clearance at lower concentrations. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Prospective studies show that up to 15% of patients taking oxaprozin chronically experience at least transient serum aminotransferase elevations. These usually resolve even with drug continuation. Marked aminotransferase elevations (>3 fold elevated) occur in approximately 1% of patients. Clinically apparent liver injury with jaundice from oxaprozin is rare (~1 per 100,000 person-years of use) and it is rarely listed in large surveys of cases of drug induced liver injury. The usual clinical presentation is an acute hepatitis-like picture arising 2 to 8 weeks after starting the medication. The pattern of injury is typically hepatocellular, but mixed hepatocellular-cholestatic cases have been described. Symptoms may include allergic manifestations such as fever, rash, arthralgias and facial edema. Autoantibody formation is rare. Liver biopsy findings are hepatocellular necrosis with prominent periportal and lobular eosinophilic infiltration suggestive of drug induced acute hepatitis. Recovery may be delayed for several days, but is usually complete within one to two months. At least one case of acute liver failure attributed to oxaprozin has been published. Likelihood score: C (probable rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Because there is no published experience with oxaprozin during breastfeeding, other agents may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding >99.5% bound to albumin Interactions Prolonged concurrent use of acetaminophen with a nonsteroidal anti-inflammatory drug may increase the risk of adverse renal effects; it is recommended that patients be under close medical supervision while receiving such combined therapy. /Nonsteroidal anti-inflammatory drugs/ Concurrent use /of alcohol or oral glucocorticoid or corticosteroids or chronic therapeutic use of corticotropin or potassium supplements/ with a nonsteroidal anti-inflammatory drug may increase the risk of gastrointestinal side effects, including ulceration or hemorrhage; however, concurrent use with a glucocorticoid or corticotropin in the treatment of arthritis may provide additional therapeutic benefit and permit reduction of glucocorticoid or corticotropin dosage. /Nonsteroidal anti-inflammatory drugs/ Increased monitoring of the response to an antihypertensive agent may be advisable when /oxaprozin/ is used concurrently because ... oxaprozin has been shown to reduce or reverse the effects of antihypertensives, possibly by inhibiting renal prostaglandin synthesis and/or by causing sodium and fluid retention. Nonsteroidal anti-inflammatory drugs may increase the hypoglycemic effect of these medications /oral antidiabetic agents or insulin/ because prostaglandins are directly involved in regulatory mechanisms of glucose metabolism and possibly because of displacement of the oral antidiabetics from serum proteins; dosage adjustments of the antidiabetic agent may be necessary; ... caution with concurrent use is recommended. /Nonsteroidal anti-inflammatory drugs/ For more Interactions (Complete) data for OXAPROZIN (8 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Dog ip 200 mg/kg LD50 Dog iv 124 mg/kg LD50 Mouse ip 376 mg/kg LD50 Mouse iv 93 mg/kg For more Non-Human Toxicity Values (Complete) data for OXAPROZIN (10 total), please visit the HSDB record page. |
||
| 参考文献 |
|
||
| 其他信息 |
Therapeutic Uses
Oxaprozin ... /is/ indicated for the treatment of acute or chronic rheumatoid arthritis. /Included in US product labeling/ Oxaprozin ... /is/ indicated for relief of acute or chronic osteoarthritis. /Included in US product labeling/ ... In this open, multicenter, randomized, controlled study, eligible patients with periarthritis of the shoulder were randomized to receive either oxaprozin 1200 mg once daily (n = 49) or diclofenac 50 mg three times daily (n = 47). The treatment period was 15 +/- 1 days. The study was planned on a hypothesis of equivalence between the two study drugs. The primary study endpoint was the change from baseline at day 15 in the patient-assessed shoulder pain score. Secondary efficacy variables included investigator-assessed shoulder function, patient-assessed quality of life on the Short-Form-36 (SF-36) Acute Health Survey and both patients' and investigators' overall assessment of efficacy. At day 15, the mean changes in shoulder pain score from baseline in the oxaprozin and diclofenac groups were -5.85 +/- SD 4.62 and -5.54 +/- SD 4.41, respectively. The difference between the two groups was not statistically significant, confirming the hypothesis of the study that oxaprozin is as effective as diclofenac. Investigator-assessed shoulder function improved in both groups but more so in the oxaprozin group (p = 0.028 at day 15). Quality of life as measured by SF-36 total score was also improved in both treatment groups, with a trend toward greater improvement in the oxaprozin group. Furthermore, a significantly more favorable effect on the SF-36 'mental health' item was observed in oxaprozin compared with diclofenac-treated patients at day 15 (p = 0.0202). As assessed by investigators, the overall efficacy of oxaprozin was superior to that for diclofenac at visit 3 (8 +/- 1 days) (p = 0.0067). Patients also assessed the overall efficacy of oxaprozin as superior to that of diclofenac at visits 3 (8 +/- 1 days) (p = 0.0235) and 4 (15 +/- 1 days) (p = 0.0272). Only six adverse events, all of which were mild or moderate in intensity and occurred in four diclofenac recipients, were observed in the study. As expected, once-daily oxaprozin proved to be as effective as diclofenac three times daily in reducing the primary efficacy variable of patient-assessed shoulder pain score in patients with periarthritis of the shoulder refractory to previous treatments with other NSAIDs. Oxaprozin was shown to be superior to diclofenac in improving shoulder function and was considered by investigators and patients to have greater overall efficacy than diclofenac. In addition, oxaprozin showed a trend toward superior results in improving patients' quality of life compared with diclofenac. A trend towards better tolerability results for oxaprozin compared with diclofenac was also noted. /EXPL THER/: The effects of eye drops containing a propionic acid derivative (oxaprozin) at 0.1% concentration on ocular inflammation produced by sodium arachidonate in the rabbit's eye were evaluated. Furthermore, the aqueous bioavailability of the drug formulation in the uninflamed and inflamed eyes was evaluated. Oxaprozin eye drops significantly reduced the signs of ocular inflammation elicited by sodium arachidonate on conjunctiva and iris. Oxaprozin treatment significantly reduced the levels of polymorphonuclear leukocytes and protein concentration in aqueous samples obtained from the eyes treated with arachidonate. Present data suggest, for the first time, that oxaprozin may be employed topically to prevent ocular reactions where the arachidonic acid cascade is activated. Drug Warnings Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. All NSAIDs, both COX-2 selective and nonselective, may have a similar risk. Patients with known cardiovascular disease or risk factors for cardiovascular disease may be at greater risk. To minimize the potential risk for an adverse cardiovascular event in patients treated with a NSAID, the lowest effective dose should be used for the shortest duration possible. Physicians and patients should remain alert for the development of such events, even in the absence of previous cardiovascular symptoms. Patients should be informed about the signs and/or symptoms of serious cardiovascular events and the steps to take if they occur. /Nonsteroidal anti-inflammatory drugs/ NSAIDs, including Daypro, can cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients, who develop a serious upper GI adverse event on NSAID therapy, is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occur in approximately 1% of patients treated for 3-6 months, and in about 2-4% of patients treated for one year. These trends continue with longer duration of use, increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short-term therapy is not without risk. NSAIDs should be prescribed with extreme caution in those with a prior history of ulcer disease or gastrointestinal bleeding. Patients with a prior history of peptic ulcer disease and/or gastrointestinal bleeding who use NSAIDs have a greater than 10-fold increased risk for developing a GI bleed compared to patients treated with neither of these risk factors. Other factors that increase the risk of GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status. Most spontaneous reports of fatal GI events are in elderly or debilitated patients and therefore, special care should be taken in treating this population. To minimize the potential risk for an adverse GI event in patients treated with an NSAID, the lowest effective dose should be used for the shortest possible duration. Patients and physicians should remain alert for signs and symptoms of GI ulcerations and bleeding during NSAID therapy and promptly initiate additional evaluation and treatment if a serious GI event is suspected. This should include discontinuation of the NSAID until a serious GI adverse event is ruled out. For high risk patients, alternate therapies that do not involve NSAIDs should be considered. /Nonsteroidal anti-inflammatory drugs/ As with other NSAIDs, anaphylactoid reactions may occur in patients without known prior exposure to Daypro. Daypro should not be given to patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs For more Drug Warnings (Complete) data for OXAPROZIN (18 total), please visit the HSDB record page. Pharmacodynamics Oxaprozin is a nonsteroidal anti-inflammatory drug (NSAID) with analgesic and antipyretic properties. Oxaprozin is used to treat rheumatoid arthritis, osteoarthritis, dysmenorrhea, and to alleviate moderate pain. |
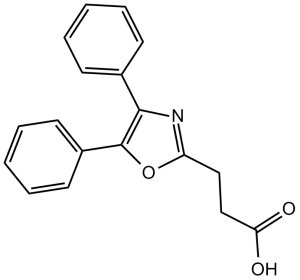
| 分子式 |
C18H15NO3
|
|
|---|---|---|
| 分子量 |
293.32
|
|
| 精确质量 |
293.105
|
|
| CAS号 |
21256-18-8
|
|
| 相关CAS号 |
Oxaprozin-d4;Oxaprozin potassium;174064-08-5;Oxaprozin-d5
|
|
| PubChem CID |
4614
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
467.0±33.0 °C at 760 mmHg
|
|
| 熔点 |
154ºC
|
|
| 闪点 |
236.2±25.4 °C
|
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
|
| 折射率 |
1.595
|
|
| LogP |
4.19
|
|
| tPSA |
63.33
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
22
|
|
| 分子复杂度/Complexity |
361
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
GSDSWSVVBLHKDQ-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25)
|
|
| 化学名 |
9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-3,7-dihydro-2H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.52 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (8.52 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (8.52 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.4092 mL | 17.0462 mL | 34.0925 mL | |
| 5 mM | 0.6818 mL | 3.4092 mL | 6.8185 mL | |
| 10 mM | 0.3409 mL | 1.7046 mL | 3.4092 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03350386 | Completed | Drug: FYU-981 Drug: Oxaprozin |
Healthy | Mochida Pharmaceutical Company, Ltd. | November 2, 2017 | Phase 1 |