| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Rapidly absorbed into the systemic circulation after intranasal administration. Bioavailability from a 400 µg dose averaged 2.8% (range 1.2 to 5.6%). Not absorbed after oral administration. Metabolism / Metabolites Enzymatic hydrolysis. Biological Half-Life 3 hours |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
Approximately 80%. |
| 参考文献 |
Ludwig C, Desmoulins PO, Driancourt MA, Goericke-Pesch S, Hoffmann B. Reversible downregulation of endocrine and germinative testicular function (hormonal castration) in the dog with the GnRH-agonist azagly-nafarelin as a removable implant 'Gonazon'; a preclinical trial. Theriogenology. 2009 Apr 15;71(7):1037-45. doi: 10.1016/j.theriogenology.2008.10.015. Epub 2009 Feb 23. PubMed PMID: 19233456.
|
| 其他信息 |
Nafarelin Acetate can cause developmental toxicity according to state or federal government labeling requirements.
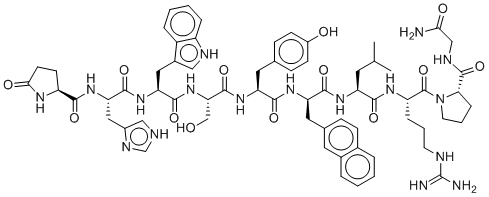
Nafarelin is a potent synthetic agonist of gonadotropin-releasing hormone with 3-(2-naphthyl)-D-alanine substitution at residue 6. Nafarelin has been used in the treatments of central precocious puberty and endometriosis. Nafarelin is a Gonadotropin Releasing Hormone Receptor Agonist. The mechanism of action of nafarelin is as a Gonadotropin Releasing Hormone Receptor Agonist. Nafarelin Acetate is the acetate salt form of nafarelin, a modified synthetic porcine luteinizing hormone (LH)-releasing hormone peptide analog, with gonadotropin-releasing hormone (GnRH) agonist activity. Upon nasal inhalation, nafarelin acetate binds to the GnRH receptor. This initially results in the release of the gonadotropins, follicle-stimulating hormone (FSH) and LH, from the pituitary gland; however, prolonged stimulation of the GnRH receptor desensitizes the receptor, which leads to decreased secretion of FSH and LH. In females, the inhibition of gonadotropin secretion causes hypogonadotropic hypogonadism leading to decreased production of estrogen and progesterone and anovulation. In males, the inhibition of LH secretion prevents the production and release of testosterone from Leydig cells in the testes and causes a significant decline in testosterone production that is near the levels seen following castration. A potent synthetic agonist of GONADOTROPIN-RELEASING HORMONE with 3-(2-naphthyl)-D-alanine substitution at residue 6. Nafarelin has been used in the treatments of central PRECOCIOUS PUBERTY and ENDOMETRIOSIS. See also: Nafarelin Acetate (has salt form). Drug Indication For treatment of central precocious puberty (true precocious puberty, GnRH-dependent precocious precocity, complete isosexual precocity) in children of both sexes and for the treatment of endometriosis. FDA Label Mechanism of Action Like GnRH, initial or intermittent administration of nafarelin stimulates release of the gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary gland, which in turn transiently increases production of estradiol in females and testosterone in both sexes. However, with continuous daily administration, nafarelin continuously occupies the GnRH receptor, leading to a reversible down-regulation of the GnRH receptors in the pituitary gland and desensitization of the pituitary gonadotropes. This causes a significant and sustained decline in the production of LH and FSH. A decline in gonadotropin production and release causes a dramatic reversible decrease in synthesis of estradiol, progesterone, and testosterone by the ovaries or testes. Like normal endometrium, endometriotic implants contain estrogen receptors. Estrogen stimulates the growth of endometrium. Use of nafarelin induces anovulation and amenorrhea and decreases serum concentrations of estradiol to the postmenopausal range, which induces atrophy of endometriotic implants. However, nafarelin does not abolish the underlying pathophysiology of endometriosis. In children with central precocious puberty receiving nafarelin, serum LH, testosterone, and estradiol concentrations return to prepubertal levels. This results in the supression of secondary sexual characteristics and decrased rate of linear growth and skeletal maturation. Following disconinuation of nafarelin, the effects of the drug is reversed, meaning FSH and LH concentrations usually return to pretreatment levels. Pharmacodynamics Nafarelin is a potent agonistic analog of gonadotropin-releasing hormone (GnRH). At the onset of administration, nafarelin stimulates the release of the pituitary gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), resulting in a temporary increase of gonadal steroidogenesis. Repeated dosing abolishes the stimulatory effect on the pituitary gland. Twice daily administration leads to decreased secretion of gonadal steroids by about 4 weeks; consequently, tissues and functions that depend on gonadal steroids for their maintenance become quiescent. After nafarelin therapy is discontinued, pituitary and ovarian function normalize and estradiol serum concentrations increase to pretreatment levels. Recurrences of endometriosis are frequent after cessation of any hormonal therapy, or surgery that leaves the ovaries and/or uterus intact. |
| 分子式 |
C66H83N17O13
|
|---|---|
| 分子量 |
1322.49
|
| 精确质量 |
1321.635
|
| CAS号 |
76932-56-4
|
| 相关CAS号 |
86220-42-0 (acetate);76932-56-4;
|
| PubChem CID |
25077405
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.5±0.1 g/cm3
|
| 折射率 |
1.711
|
| LogP |
0.89
|
| tPSA |
472.13
|
| 氢键供体(HBD)数目 |
16
|
| 氢键受体(HBA)数目 |
15
|
| 可旋转键数目(RBC) |
33
|
| 重原子数目 |
96
|
| 分子复杂度/Complexity |
2730
|
| 定义原子立体中心数目 |
9
|
| SMILES |
O=C([C@]([H])(C([H])([H])C([H])([H])C([H])([H])/N=C(\N([H])[H])/N([H])[H])N([H])C([C@]([H])(C([H])([H])C([H])(C([H])([H])[H])C([H])([H])[H])N([H])C([C@@]([H])(C([H])([H])C1C([H])=C([H])C2=C([H])C([H])=C([H])C([H])=C2C=1[H])N([H])C([C@]([H])(C([H])([H])C1C([H])=C([H])C(=C([H])C=1[H])O[H])N([H])C([C@]([H])(C([H])([H])O[H])N([H])C([C@]([H])(C([H])([H])C1=C([H])N([H])C2=C([H])C([H])=C([H])C([H])=C12)N([H])C([C@]([H])(C([H])([H])C1=C([H])N=C([H])N1[H])N([H])C([C@]1([H])C([H])([H])C([H])([H])C(N1[H])=O)=O)=O)=O)=O)=O)=O)=O)N1C([H])([H])C([H])([H])C([H])([H])[C@@]1([H])C(N([H])C([H])([H])C(N([H])[H])=O)=O
|
| InChi Key |
(S)-1-(((R)-2-((S)-2-((S)-2-((S)-2-((S)-3-(1H-imidazol-4-yl)-2-((S)-5-oxopyrrolidine-2-carboxamido)propanamido)-3-(1H-indol-3-yl)propanamido)-3-hydroxypropanamido)-3-(4-hydroxyphenyl)propanamido)-3-(naphthalen-2-yl)propanoyl)-L-leucyl-L-arginyl)-N-(2-amino-2-oxoethyl)pyrrolidine-2-carboxamide
|
| InChi Code |
RWHUEXWOYVBUCI-ITQXDASVSA-N SMILES
|
| 化学名 |
Nafarelin
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.7561 mL | 3.7807 mL | 7.5615 mL | |
| 5 mM | 0.1512 mL | 0.7561 mL | 1.5123 mL | |
| 10 mM | 0.0756 mL | 0.3781 mL | 0.7561 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。