| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
α2-adrenergic receptor ( pKi = 6.95 ); 5-HT3 Receptor ( pKi = 8.1 ); 5-HT2 Receptor ( pKi = 8.05 ); H1 Receptor ( pKi = 9.3 )
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:米氮平对克隆的人 α2A-肾上腺素 (AR) 受体表现出显着的亲和力,可阻断去甲肾上腺素 (NA) 诱导的鸟苷-5-O-(3-[35S]硫代)-三磷酸 ([35S] ]-GTPgammaS) 结合。米氮平对克隆的人血清素 (5-HT)2C 受体表现出高亲和力,可消除 5-HT 诱导的磷酸肌醇生成。米氮平显着提高透析液中 NA 的水平,在 FCX 中,提高 DA 的水平,而 5-HT 不受影响。米氮平通过阻断 α2 肾上腺素能自身受体和异质受体来增强 5-HT 上行通路电刺激的有效性。米氮平可阻断微离子导入去甲肾上腺素 (NE) 对 CA3 背侧海马锥体神经元放电活动的抑制作用,表明它们对突触后 α-2 肾上腺素受体具有拮抗作用。
|
| 体内研究 (In Vivo) |
Mirtazapine (10-250 mg/kg iv) 可以剂量依赖性地增强初始大鼠中 5-HT 神经元的放电活性,但在 6-羟基多巴胺预处理的大鼠中则不然。米氮平(5 mg/kg/天,皮下注射,使用微型渗透泵)可增加雄性 Sprague-Dawley 大鼠蓝斑去甲肾上腺素 (NA) 神经元的自发放电活动。米氮平可拮抗低剂量(10 mg/kg,静脉注射)α2-肾上腺素受体激动剂可乐定对电刺激有效性的增强作用和高剂量(100 mg/kg,静脉注射)的降低作用。上行 5-HT 通路抑制背侧海马 CA3 锥体神经元的放电活动。 Mirtazapine (5 mg/kg sc) 仅轻微影响纹状体中的 DOPAC 和高香草酸水平,几乎不影响自由活动大鼠的 5-HT 释放,但明显增加 5-羟基吲哚乙酸。
|
| 酶活实验 |
比较了1,2,3,4,10,14b-六氢-2-甲基吡嗪并[2,1-a]吡啶并[2,3-c][2]苯并氮杂卓[+/-)Org 3770和相关抗抑郁药物mianserin的神经化学和自主药理学特征。与mianserin(pKi=7.4)相比,Org 3777(+/-)对[3H]去甲肾上腺素([3H]NA)的体外摄取影响较弱(pKi=5.6)。(+/-)Org 3770和米安色林都促进了皮质切片中[3H]NA的释放。α2肾上腺素受体介导的NA对[3H]NA或[3H]血清素([3H]5-HT)释放的影响被(+)Org 3770拮抗,pKi值分别为8.4和8.1。然而,(-)Org 3770仅拮抗NA对[3H]5-HT释放的影响(pA2=7.7)。(+/-)Org 3770和米安色林以相同的亲和力(pKi=7.0)抑制了[3H]劳沃尔辛与α2-肾上腺素受体的结合,而(+/-”Org 3777”(pKi=6.4)对[3H]哌唑嗪与α1-肾上腺素受体结合的影响小于米安色琳”(pKi=7.1)。在大鼠输精管中的α1-和α2-肾上腺素受体也发现了类似的差异。(+/-)Org 3770(pKi=8.1)对[3H]米安色林与5-HT2受体的结合的阻断作用不如米安色林强(pKi=4.4),而(+/-”Org 3777(pKi=9.3)对[3K]美吡拉敏与组胺-1受体的结合作用比米安色兰强(pKi=8.75)。[3H]奎核环烷基苯甲酸酯与毒蕈碱胆碱能受体的结合被(+/-)Org 3770(pKi=6.1)和米安色林(pKi=6.3)同等阻断。在分离器官中获得了类胰蛋白酶-D、组胺-1和毒蕈碱胆碱能受体的类似数据。阻断α2肾上腺素受体在米安色林和(+/-)Org 3770治疗抑郁症的疗效中起着重要作用,可能排除了抑制NA摄取的作用[2]。
|
| 细胞实验 |
米氮平对人单核细胞和CD4 T细胞在体外产生细胞因子/趋化因子的影响[3]
使用autoMACS分离机和autoMACS CD14+阳性选择试剂盒从健康供体外周血中分离CD14+单核细胞。将CD14+细胞接种到添加了10%FBS、1 mM丙酮酸钠、2 mM l-谷氨酰胺和100单位/ml青霉素和链霉素以及非必需氨基酸(NEAA)的500μl RPMI 1640培养基中的24孔组织培养板(密度为1×106个细胞/孔)中。孵育4小时(5%CO2,37°C)后,通过洗涤去除非贴壁细胞,并将500μl预热的完全新鲜培养基加入孔中。指定的孔用米氮平(10μM)或赋形剂(0.2μl/ml DMSO)处理。一小时后,将Con A(5μg/ml)或载体加入指定的孔中,细胞再培养24小时。收集上清液并储存在-80°C下,直至检测细胞因子/趋化因子水平(以pg/ml表示) 使用EasySep™人CD4+T细胞分离试剂盒从健康供体外周血中分离CD4+T细胞。流式细胞术检测的分离细胞纯度>97%。细胞在24孔板(密度106个细胞/孔)中在500μl RPMI 1640培养基中培养,该培养基补充了10%FBS、1 mM丙酮酸钠、2 mM l-谷氨酰胺和100单位/ml青霉素和链霉素以及非必需氨基酸(NEAA)。指定的孔用米氮平(10μM)或赋形剂(0.2μl/ml DMSO)处理。一小时后,将Con A(5μg/ml)或载体加入指定的孔中,细胞再培养24小时。收集上清液并储存在-80°C下,直至检测细胞因子水平。根据制造商的方案,使用人MILLIPEX试剂盒在培养上清液中测量人IL-10、IL-4和IFNγ。使用Luminex 100系统进行多路复用分析 。 |
| 动物实验 |
Male C57BL/6 mice (8-10 week old) treated with concanavalin A (Con A)
1 mg/kg, 10 mg/kg, and 20 mg/kg Intraperitoneal injection; once Mirtazapine Treatment and Con A Hepatitis Severity[3] To delineate the impact of mirtazapine treatment in Con A hepatitis, mice were treated 1 h prior to Con A treatment with mirtazapine 1–20 mg/kg intraperitoneally (ip). Blood and liver samples were collected under isoflurane anesthesia 16 h post-Con A treatment (unless otherwise noted) to assess liver injury biochemically (plasma alanine aminotransferase [ALT] activity; measured using Roche-Hitachi Modular-P800 apparatus) and histologically using formalin-fixed liver tissue slices stained with Hematoxylin and Eosin (H&E). Extent of liver parenchymal necrosis was quantitated as previously described using Image J software and an Olympus XC10 camera (acquired using the Olympus VS-ASW software package; original magnification x400). In additional experiments, mirtazapine (20 mg/kg ip) was administered 2 h after Con A treatment (i.e., therapeutically) and mice sacrificed 16 h later and severity of liver injury determined by ALT measurement. In further experiments, the impact of specifically blocking individual receptors known to be impacted by mirtazapine treatment (i.e., 5HT2a, 5HT2c, 5HT3, and H1; also 5HT1a receptor) on the severity of Con A hepatitis was determined by ALT measurement. |
| 药代性质 (ADME/PK) |
Absorption
The absorption of this drug is rapid and complete. Due to first pass metabolism in the liver and metabolism in the gut wall, absolute bioavailability is about 50%. Peak blood concentrations are attained within about 2 hours after an oral dose. Food has little effect on the absorption of mirtazapine, and no dose adjustment is required if it is taken with food. Steady-state levels are achieved by about 5 days after the initial dose. Mirtazapine pharmacokinetics vary across gender and age range. Females and the elderly population have been shown to have higher blood concentrations in comparison to males and younger adults. Route of Elimination This drug is mainly excreted by the kidney. It is 75% eliminated in the urine and 15% eliminated in the feces. Volume of Distribution The volume of distribution after an oral steady-state dose was measured to be 107 ± 42L in a pharmacokinetic study. Clearance Total body clearance in males was found to be 31 L/h in a clinical pharmacokinetics study after intravenous administration. **Clearance in elderly patients* Mirtazapine clearance is slower in the elderly than in younger subjects. Exercise caution when this drug is given to elderly patients. In a clinical trial, elderly males showed a marked decrease in mirtazapine clearance when compared to young males taking the same dose. This difference was less significant when clearance was compared between elderly females and younger females taking mirtazapine. **Clearance in hepatic and renal impairment** Patients with hepatic and renal impairment have decreased rates of clearance and dosage adjustments may be necessary for these patients. Moderate renal impairment and hepatic impairment cause about a 30% decrease in mirtazapine clearance. Severe renal impairment leads to a 50% decrease in mirtazapine clearance. Metabolism / Metabolites Mirtazapine is heavily metabolized in humans. Demethylation and hydroxylation and subsequent glucuronide conjugation are the major pathways by which mirtazapine is metabolized. Data from in vitro studies on human liver microsomes show that cytochrome 2D6 and 1A2 lead to the formation of the _8-hydroxy metabolite_ of mirtazapine. The CYP3A enzyme metabolizes this drug into its _N-desmethyl and N-oxide_ metabolites. There are various other unconjugated metabolites of this drug that are pharmacologically active, but are measured in the blood at limited concentrations. Mirtazapine has known human metabolites that include Mirtazapine N-oxide, N-Desmethylmirtazapine, and 8-hydroxy-mirtazapine. Mirtazapine is extensively metabolized by demethylation and hydroxylation followed by glucuronide conjugation. Cytochrome P450 2D6 and cytochrome P450 1A2 are involved in formation of the 8-hydroxy metabolite of mirtazapine, and cytochrome P450 3A4 is responsible for the formation of the N-desmethyl and N-oxide metabolites. Several metabolites possess pharmacological activity, but plasma levels are very low. Route of Elimination: This drug is known to be substantially excreted by the kidney (75%). Half Life: 20-40 hours Toxin and Toxin Target Database (T3DB) Biological Half-Life: 20-40 hours |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Mirtazapine acts as an antagonist at central pre-synaptic alpha(2)-receptors, inhibiting negative feedback to the presynaptic nerve and causing an increase in NE release. Blockade of heteroreceptors, alpha(2)-receptors contained in serotenergic neurons, enhances the release of 5-HT, increasing the interactions between 5-HT and 5-HT1 receptors and contributing to the anxiolytic effects of mirtazapine. Mirtazapine also acts as a weak antagonist at 5-HT1 receptors and as a potent antagonist at 5-HT2 (particularly subtypes 2A and 2C) and 5-HT3 receptors. Blockade of these receptors may explain the lower incidence of adverse effects such as anxiety, insomnia, and nausea. Mirtazapine also exhibits significant antagonism at H1-receptors, resulting in sedation. Mirtazapine has no effects on the reuptake of either NE or 5-HT and has only minimal activity at dopaminergic and muscarinic receptors. Hepatotoxicity Liver test abnormalities have been reported to occur in up to 10% of patients on mirtazapine, but elevations are usually modest and rarely require dose modification or discontinuation. Rare instances of acute, clinically apparent episodes of liver injury with marked liver enzyme elevations with or without jaundice have been reported in patients on mirtazapine. The onset of injury has varied greatly from several months to several years. The pattern of serum enzyme elevations is usually hepatocellular, but mixed forms have also been described. Autoimmune (autoantibodies) and immunoallergic features (rash, fever, eosinophilia) are uncommon. Likelihood score: C (probable rare cause of clinically apparent liver disease). Toxicity Data LD50: 600-720mg/kg (oral, mice) (L1855) LD50: 320-490mg/kg (oral, rat) (L1855) View More
Treatment Protein Binding Mirtazapine is about 85% bound to plasma proteins. Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited information indicates that maternal doses of up to 120 mg daily produce low levels in milk and would not be expected to cause any adverse effects in breastfed infants, especially if the infant is older than 2 months. If mirtazapine is required by the mother, it is not a reason to discontinue breastfeeding. A safety scoring system finds mirtazapine use to be possible during breastfeeding. Exclusively breastfed infants should be monitored for behavioral side effects and adequate growth if this drug is used during lactation. ◉ Effects in Breastfed Infants A 14-week old infant was breastfed 6 times daily during maternal treatment with mirtazapine 30 mg daily. After 6 weeks of therapy, the infant was judged to have normal psychomotor development and no adverse effects, including sedation or abnormal weight gain. Eight infants who averaged 6.3 months of age (range 1.5 to 13 months) were breastfed by mothers taking mirtazapine in an average dosage of 495 mcg/kg daily for 6 to 129 days. At the time they were studied, the average Denver developmental age in the 7 infants studied averaged 101% of normal. No adverse effects were noted in any of the infants. A 6-week-old infant was exclusively breastfed by a mother who was taking mirtazapine 22.5 mg at daily at night beginning at 4 weeks postpartum. Weekly follow-ups of the infant found no sedation or alterations in weight gain, although the infant's weight was consistently below the 25th percentile even before mirtazapine was begun. A 2-month-old infant was breastfed (extent not stated) by a mother taking 15 mg of mirtazapine daily during pregnancy and lactation. The mother stated that the infant had a higher birthweight and gained weight more rapidly than her previous 3 infants, and that unlike her other infants, this infant slept through the night at this age. The authors noted that these observations cannot necessarily be attributed to mirtazapine. In a case series of 55 women who took mirtazapine during pregnancy and postpartum, 24 of their 56 infants were exclusively breastfed and 20 were partially breastfed. Of the infants who were exposed in utero during the third trimester, those who were breastfed either partially or exclusively had a lower frequency of poor neonatal adaptation syndrome than those who were exclusively formula fed. No sleeping or feeding problems were seen in any of the infants. A case series reported 8 women who received paroxetine 20 mg and mirtazapine 15 mg daily for various psychiatric disorders. The women breastfed (extent not stated) their infants who averaged 4.3 weeks of age. Follow-up of the infants after 3 to 6 weeks when mirtazapine was discontinued found one infant who experienced restlessness after 5 days of therapy according to the mother. Discontinuation of mirtazapine had no effect, but the symptoms disappeared when paroxetine was discontinued. No other infants had other adverse effects observed. ◉ Effects on Lactation and Breastmilk Gynecomastia, hyperprolactinemia and galactorrhea were reported in an 89-year-old man after 21 months of therapy with mirtazapine 30 mg daily. Symptoms regressed 1 month after discontinuation and prolactin levels normalized. A 28-year-old female inpatient developed galactorrhea, mastodynia, fatigue, and extended subcutaneous edema of the trunk and extremities 4 weeks after adjusting her mirtazapine dose to 30 mg daily. At this time her morning serum prolactin level was not elevated; however, 12 days later, morning serum prolactin was 32.1 mcg/L (normal range 4.79-23.3 mcg/L). Serum prolactin normalized, and edema and galactorrhea remitted within 1 week after discontinuing mirtazapine and starting escitalopram. Pituitary tumor was ruled out. Mirtazapine was probably the cause of the patient's symptoms. The clinical relevance of these findings in nursing mothers is not known. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. An observational study looked at outcomes of 2859 women who took an antidepressant during the 2 years prior to pregnancy. Compared to women who did not take an antidepressant during pregnancy, mothers who took an antidepressant during all 3 trimesters of pregnancy were 37% less likely to be breastfeeding upon hospital discharge. Mothers who took an antidepressant only during the third trimester were 75% less likely to be breastfeeding at discharge. Those who took an antidepressant only during the first and second trimesters did not have a reduced likelihood of breastfeeding at discharge. The antidepressants used by the mothers were not specified. A retrospective cohort study of hospital electronic medical records from 2001 to 2008 compared women who had been dispensed an antidepressant during late gestation (n = 575; mirtazapine n = 12) to those who had a psychiatric illness but did not receive an antidepressant (n = 1552) and mothers who did not have a psychiatric diagnosis (n = 30,535). Women who received an antidepressant were 37% less likely to be breastfeeding at discharge than women without a psychiatric diagnosis, but no less likely to be breastfeeding than untreated mothers with a psychiatric diagnosis. In a study of 80,882 Norwegian mother-infant pairs from 1999 to 2008, new postpartum antidepressant use was reported by 392 women and 201 reported that they continued antidepressants from pregnancy. Compared with the unexposed comparison group, late pregnancy antidepressant use was associated with a 7% reduced likelihood of breastfeeding initiation, but with no effect on breastfeeding duration or exclusivity. Compared with the unexposed comparison group, new or restarted antidepressant use was associated with a 63% reduced likelihood of predominant, and a 51% reduced likelihood of any breastfeeding at 6 months, as well as a 2.6-fold increased risk of abrupt breastfeeding discontinuation. Specific antidepressants were not mentioned. ◈ What is mirtazapine? Mirtazapine is a medication that has been used to treat major depressive disorder and severe vomiting/nausea during pregnancy (hyperemesis gravidarum). A brand name for mirtazapine is Remeron®. MotherToBaby has fact sheets on depression https://mothertobaby.org/fact-sheets/depression-pregnancy/ and nausea and vomiting https://mothertobaby.org/fact-sheets/nausea-vomiting-pregnancy-nvp/.Sometimes when people find out they are pregnant, they think about changing how they take their medication, or stopping their medication altogether. However, it is important to talk with your healthcare providers before making any changes to how you take your medication. Your healthcare providers can talk with you about the benefits of treating your condition and the risks of untreated illness during pregnancy. Some people may have a return of their symptoms (relapse) if they stop this medication during pregnancy. ◈ I take mirtazapine. Can it make it harder for me to get pregnant? In some people, mirtazapine may raise the levels of a hormone called prolactin. High levels of prolactin can stop ovulation (part of the menstrual cycle when an ovary releases an egg). This would make it harder to get pregnant. Your healthcare provider can test your levels of prolactin if there is concern. ◈ Does taking mirtazapine increase the chance of miscarriage? Miscarriage is common and can occur in any pregnancy for many different reasons. One study found no link between the use of mirtazapine and an increased chance of miscarriage. Depression itself might increase the chance for miscarriage. ◈ Does taking mirtazapine increase the chance of birth defects? Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Studies and case reports have looked at nearly 5,000 pregnancies with mirtazapine exposure and have not found an increased chance of birth defects. ◈ Does taking mirtazapine in pregnancy increase the chance of other pregnancy-related problems? Some studies suggest that taking mirtazapine throughout pregnancy might increase the chance of other pregnancy-related problems, such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth). Research has also shown that when depression is left untreated during pregnancy, there could be an increased chance for pregnancy complications such as preterm delivery, low birth weight, and pre-eclampsia (high blood pressure and problems with organs, such as the kidneys) that can lead to seizures (called eclampsia). This makes it hard to know if it is a medication, an underlying condition, or other factors that are increasing the chance of these problems. ◈ I need to take mirtazapine throughout my entire pregnancy. Will it cause withdrawal symptoms in my baby after birth? The use of mirtazapine during pregnancy can cause temporary symptoms in newborns soon after birth. These symptoms are sometimes referred to as withdrawal. Symptoms might include sensitivity to light and sound (called excitability), fast heart rate, tremors, and problems regulating body temperature shortly after birth. In most cases, these symptoms are mild and go away on their own. Some babies may need to stay in a special care nursery until the symptoms go away. Not all babies exposed to mirtazapine will have these symptoms. It is important that your healthcare providers know you are taking mirtazapine so that if symptoms occur your baby can get the care that’s best for them. ◈ Does taking mirtazapine in pregnancy affect future behavior or learning for the child? Studies have not been done to see if mirtazapine can cause behavior or learning issues for the child. ◈ Breastfeeding while taking mirtazapine: Mirtazapine gets into breastmilk in small amounts. Most nursing babies have not had reported side effects from the medication in breast milk. If you suspect the baby has any symptoms (being overly sleepy) contact the child’s healthcare provider. Be sure to talk to your healthcare provider about all your breastfeeding questions. ◈ If a male takes mirtazapine, could it affect fertility or increase the chance of birth defects? It is not known if mirtazapine could affect male fertility (ability to get partner pregnant) or increase the chance of birth defects above the background risk. In general, exposures that fathers or sperm donors have are unlikely to increase risks to a pregnancy. For more information, please see the MotherToBaby fact sheet Paternal Exposures at https://mothertobaby.org/fact-sheets/paternal-exposures-pregnancy/. Exposure Routes Oral. Rapid and complete, but, due to first-pass metabolism, absolute bioavailability is 50%. Toxin and Toxin Target Database (T3DB) Symptoms Symptoms of overdose include disorientation, drowsiness, impaired memory, and tachycardia. |
| 参考文献 |
|
| 其他信息 |
Mirtazapine is a benzazepine and a tetracyclic antidepressant. It has a role as an alpha-adrenergic antagonist, a serotonergic antagonist, a histamine antagonist, an anxiolytic drug, a H1-receptor antagonist and a oneirogen.
Mirtazapine is a tetracyclic piperazino-azepine antidepressant agent that was initially approved for the treatment of major depressive disorder (MDD) in the Netherlands in 1994. This drug was first manufactured by Organon Inc., and received FDA approval in 1997 for the treatment of major depressive disorder. The effects of this drug may be observed as early as 1 week after beginning therapy. In addition to its beneficial effects in depression, mirtazapine has been reported to be efficacious in the off-label management of various other conditions. It may improve the symptoms of neurological disorders, reverse weight loss caused by medical conditions, improve sleep, and prevent nausea and vomiting after surgery. Mirtazapine is a tetracyclic antidepressant with a somewhat unique mechanism of action. Mirtazapine therapy can be associated with transient asymptomatic elevations in serum aminotransferase levels and has been linked to rare instances of clinically apparent acute liver injury. Mirtazapine is a synthetic tetracyclic derivative of the piperazino-azepines with antidepressant activity. Although its mechanism of action is unknown, mirtazapine enhances central adrenergic and serotonergic transmission, possibly by acting as an antagonist at central presynaptic alpha 2 adrenergic inhibitory autoreceptors and heteroreceptors. This agent is a potent antagonist of 5-hydroxytryptamine type 2 (5-HT2), 5-HT3, and histamine 1 (H1) receptors, and a moderate antagonist of peripheral alpha 1 adrenergic and muscarinic receptors. Mirtazapine is an antidepressant introduced by Organon International in 1996 used for the treatment of moderate to severe depression. Mirtazapine has a tetracyclic chemical structure and is classified as a noradrenergic and specific serotonergic antidepressant (NaSSA). It is the only tetracyclic antidepressant that has been approved by the Food and Drug Administration to treat depression. A piperazinoazepine tetracyclic compound that enhances the release of NOREPINEPHRINE and SEROTONIN through blockage of presynaptic ALPHA-2 ADRENERGIC RECEPTORS. It also blocks both 5-HT2 and 5-HT3 serotonin receptors and is a potent HISTAMINE H1 RECEPTOR antagonist. It is used for the treatment of depression, and may also be useful for the treatment of anxiety disorders. View More
Drug Indication Pharmacodynamics **General effects and a note on suicidality** Mirtazapine is effective in treating moderate to severe depression and treats many symptoms normally associated with this condition. These symptoms may include disturbed sleep, lack of appetite, and anhedonia, in addition to anxiety.. It is important to note that suicidal ideation and behavior may emerge or increase during treatment with mirtazapine, as with any other antidepressant. This risk is especially pronounced in younger individuals. Patients, medical professionals, and families should monitor for suicidal thoughts, worsening depression, anxiety, agitation, sleep changes, irritable behavior, aggression, impulsivity, restlessness, and other unusual behavior when this drug is taken or the dose is adjusted. Do not administer mirtazapine to children. When deciding to prescribe this drug, carefully consider the increased risk of suicidal thoughts and behavior, especially in young adults. **Effects on appetite and weight gain** In addition to the above effects, mirtazapine exerts stimulating effects on appetite, and has been used for increasing appetite and decreasing nausea in cancer patients. Some studies and case reports have shown that this drug improves eating habits and weight gain in patients suffering from anorexia nervosa when administered in conjunction with psychotherapy and/or other psychotropic drugs. In a clinical trial, women with depression experienced a clinically significant mean increase in body weight, fat mass, and concentrations of leptin when treated with mirtazapine for a 6-week period, with a lack of effect on glucose homeostasis. **Effects on sleep** The use of mirtazapine to treat disordered sleep has been leveraged from its tendency to cause somnolence, which is a frequently experienced adverse effect by patients taking this drug. Mirtazapine has been shown to exert beneficial effects on sleep latency, duration, and quality due to its sedating properties. Insomnia is a common occurrence in patients with depression, and mirtazapine has been found to be efficacious in treating this condition. Mechanism of Action **Summary** The mechanism of action of mirtazapine is not fully understood but may be explained by its effects on central adrenergic and serotonergic activity. This drug exhibits a fast onset of action, a high level of response, a manageable side-effect profile, and dual noradrenergic and serotonergic effects that are unique from the effects of other antidepressants. **Effects on various receptors** It has been shown that both noradrenergic and serotonergic activity increase following mirtazapine administration. The results of these studies demonstrate mirtazapine exerts antagonist activity at presynaptic α2-adrenergic inhibitory autoreceptors and heteroreceptors in the central nervous system. This is thought to lead to enhanced noradrenergic and serotonergic activity, which are known to improve the symptoms of depression and form the basis of antidepressant therapy. Mirtazapine is a strong antagonist of serotonin 5-HT2 and 5-HT3 receptors. It has not been found to bind significantly to the serotonin 5-HT1A and 5-HT1B receptors but indirectly increases 5-HT1A transmission. In addition to the above effects, mirtazapine is a peripheral α1-adrenergic antagonist. This action may explain episodes of orthostatic hypotension that have been reported after mirtazapine use. Mirtazapine is a potent histamine (H1) receptor antagonist, which may contribute to its powerful sedating effects. The pain-relieving effects of mirtazapine may be explained by its effects on opioid receptors. The novel antidepressant mirtazapine has a dual mode of action. It is a noradrenergic and specific serotonergic antidepressant (NaSSA) that acts by antagonizing the adrenergic alpha2-autoreceptors and alpha2-heteroreceptors as well as by blocking 5-HT2 and 5-HT3 receptors. It enhances, therefore, the release of norepinephrine and 5-HT1A-mediated serotonergic transmission. This dual mode of action may conceivably be responsible for mirtazapine's rapid onset of action. Mirtazapine is extensively metabolized in the liver. The cytochrome (CYP) P450 isoenzymes CYP1A2, CYP2D6, and CYP3A4 are mainly responsible for its metabolism. Using once daily dosing, steady-state concentrations are reached after 4 days in adults and 6 days in the elderly. In vitro studies suggest that mirtazapine is unlikely to cause clinically significant drug-drug interactions. Dry mouth, sedation, and increases in appetite and body weight are the most common adverse effects. In contrast to selective serotonin reuptake inhibitors (SSRIs), mirtazapine has no sexual side effects. The antidepressant efficacy of mirtazapine was established in several placebo-controlled trials. In major depression, its efficacy is comparable to that of amitriptyline, clomipramine, doxepin, fluoxetine, paroxetine, citalopram, or venlafaxine. Mirtazapine also appears to be useful in patients suffering from depression comorbid with anxiety symptoms and sleep disturbance. It seems to be safe and effective during long-term use.[1] Activation of the innate immune system, including tissue macrophages and associated neutrophil infiltration, is an important driver of subsequent adaptive immune responses in many autoimmune diseases, including autoimmune hepatitis (AIH). The antidepressant mirtazapine has a unique complex pharmacology, altering signaling through a number of serotonin and histamine receptors that can impact macrophage function; an effect potentially influencing AIH outcome. In the mouse model of concanavalin A (Con A) induced liver injury (mimics many aspects of human AIH), in which early innate immune activation (i.e., stimulated hepatic macrophages/monocytes recruit neutrophils and additional monocytes to the liver) critically drives immune-mediated hepatitis induction, mirtazapine strikingly and dose-dependently inhibited Con A-induced liver injury. This inflammation-suppressing effect of mirtazapine was linked to an attenuation of Con A-stimulated early innate immune responses within the liver, including inhibition of hepatic macrophage/monocyte activation, decreased hepatic macrophage/monocyte-derived pro-inflammatory cytokine (e.g., TNFα) and chemokine (e.g., CXCL1 and CXCL2) production, suppression of Con A-induced increases in the hepatic expression of the neutrophil relevant endothelial cell adhesion molecule ICAM-1, with the resultant significant reduction in neutrophil recruitment into the liver. Consistent with our findings in the Con A model, mirtazapine also significantly reduced activation-induced release of cytokine/chemokine mediators from human CD14+ monocytes in vitro. Conclusion: Our data suggest that mirtazapine can attenuate hepatic innate immune responses that critically regulate the subsequent development of autoimmune liver injury. Therefore, given that it is a safe and widely used medication, mirtazapine may represent a novel therapeutic approach to autoimmune liver disease.[3] |
| 分子式 |
C17H19N3
|
|
|---|---|---|
| 分子量 |
265.35
|
|
| 精确质量 |
265.157
|
|
| 元素分析 |
C, 76.95; H, 7.22; N, 15.84
|
|
| CAS号 |
85650-52-8
|
|
| 相关CAS号 |
(S)-Mirtazapine; 61337-87-9; (S)-Mirtazapine-d3; (R)-Mirtazapine; 61364-37-2; Mirtazapine-d3; 1216678-68-0; Mirtazapine-d4; 1215898-55-7; (R)-Mirtazapine-d3;
85650-52-8; 61337-67-5 (deleted); 1448014-35-4 (HCl); 207516-99-2 (HCl); 207516-99-2 (2HCl); 868363-97-7 (HBr); 868528-74-9 (HBr); 341512-89-8 (hemihydrate)
|
|
| PubChem CID |
4205
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
432.4±45.0 °C at 760 mmHg
|
|
| 熔点 |
114-116ºC
|
|
| 闪点 |
215.3±28.7 °C
|
|
| 蒸汽压 |
0.0±1.0 mmHg at 25°C
|
|
| 折射率 |
1.668
|
|
| LogP |
2.75
|
|
| tPSA |
19.37
|
|
| SMILES |
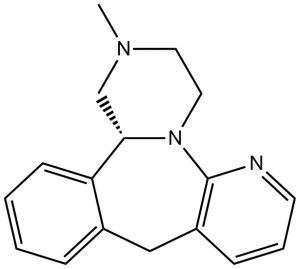
N1C2N3C(C4C(CC=2C=CC=1)=CC=CC=4)CN(C)CC3
|
|
| InChi Key |
RONZAEMNMFQXRA-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C17H19N3/c1-19-9-10-20-16(12-19)15-7-3-2-5-13(15)11-14-6-4-8-18-17(14)20/h2-8,16H,9-12H2,1H3
|
|
| 化学名 |
5-methyl-2,5,19-triazatetracyclo[13.4.0.02,7.08,13]nonadeca-1(15),8,10,12,16,18-hexaene
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (9.42 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (9.42 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (9.42 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7686 mL | 18.8430 mL | 37.6861 mL | |
| 5 mM | 0.7537 mL | 3.7686 mL | 7.5372 mL | |
| 10 mM | 0.3769 mL | 1.8843 mL | 3.7686 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00488072 | Active Recruiting |
Drug: Mirtazapine Drug: Placebo |
Advanced Cancer Anorexia Weight Loss Insomnia |
M.D. Anderson Cancer Center | September 20, 2006 | Phase 2 |
| NCT03935685 | Recruiting | Drug: Mirtazapine (Remeron) |
Glioma of Brain | University of California, Irvine | February 26, 2019 | Phase 2 |
| NCT05380479 | Recruiting | Drug: Mirtazapine Drug: Megestrol Acetate |
Anorexia | Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh |
June 1, 2022 | Phase 2 |
| NCT04614584 | Recruiting | Drug: Mirtazapine Drug: Methamphetamine |
Cardiovascular Abnormalities Drug Interaction |
San Francisco Department of Public Health |
July 12, 2021 | Phase 1 |
| NCT04728581 | Not yet recruiting | Drug: Mirtazapine Drug: Quetiapine Drug: Placebo |
Insomnia Osteo Arthritis Knee |
St. Anna Ziekenhuis, Geldrop, Netherlands |
January 1, 2024 | Not Applicable |
 |
|---|