| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|

| 体外研究 (In Vitro) |
体外活性:米那普仑主要以母体和葡萄糖苷酸形式从尿中排出(> 80%),只有一小部分(< 10%)通过 CYP3A4 酶的 N-脱乙基化代谢。高浓度的Milnacipran可抑制某些配体门控离子通道(LGIC)受体,包括NMDA、5-HT3A和nACh受体,IC50分别为58.4 μM、185 μM、14.3 μM。
|
||
|---|---|---|---|
| 体内研究 (In Vivo) |
米那普仑(10 和 30 mg/kg,PO)会导致大鼠内侧前额叶皮层细胞外 5-HT 和 NA 水平与剂量相关的增加。米那普仑(30 和 60 mg/kg,PO)显着缩短了大鼠强迫游泳试验中不动时间和条件性恐惧应激试验中冻结时间的持续时间,大鼠分别是抑郁和焦虑的动物行为模型。 Milnacipran (<40 mg/kg ip) 剂量依赖性地增加自由活动的豚鼠下丘脑中 NA 和 5-HT 的细胞外水平。在自由活动的豚鼠下丘脑中,给予 10 mg/kg 和 40 mg/kg 的米那普仑,NA 代谢物 MHPG 水平分别降低 57% 和 47%。
|
||
| 动物实验 |
|
||
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Amounts of milnacipran in breastmilk are low and would not be expected to cause any adverse effects in breastfed infants. However, until more data become available, milnacipran should be used with caution during breastfeeding, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Galactorrhea is reported by the manufacturer to be a side effect of milnacipran. One woman who was being treated for depression took an intentional overdose of 950 mg of milnacipran orally. From day 5 to day 15 after the overdose, the patient noted a flow of milk from her left breast. The galactorrhea resolved without treatment. In a study of cases of hyperprolactinemia and its symptoms (e.g., gynecomastia) reported to a French pharmacovigilance center, milnacipran was not found to have an increased risk of causing hyperprolactinemia compared to other drugs. An observational study looked at outcomes of 2859 women who took an antidepressant during the 2 years prior to pregnancy. Compared to women who did not take an antidepressant during pregnancy, mothers who took an antidepressant during all 3 trimesters of pregnancy were 37% less likely to be breastfeeding upon hospital discharge. Mothers who took an antidepressant only during the third trimester were 75% less likely to be breastfeeding at discharge. Those who took an antidepressant only during the first and second trimesters did not have a reduced likelihood of breastfeeding at discharge. The antidepressants used by the mothers were not specified. A retrospective cohort study of hospital electronic medical records from 2001 to 2008 compared women who had been dispensed an antidepressant during late gestation (n = 575) to those who had a psychiatric illness but did not receive an antidepressant (n = 1552) and mothers who did not have a psychiatric diagnosis (n = 30,535). Women who received an antidepressant were 37% less likely to be breastfeeding at discharge than women without a psychiatric diagnosis, but no less likely to be breastfeeding than untreated mothers with a psychiatric diagnosis. None of the mothers were taking milnacipran. In a study of 80,882 Norwegian mother-infant pairs from 1999 to 2008, new postpartum antidepressant use was reported by 392 women and 201 reported that they continued antidepressants from pregnancy. Compared with the unexposed comparison group, late pregnancy antidepressant use was associated with a 7% reduced likelihood of breastfeeding initiation, but with no effect on breastfeeding duration or exclusivity. Compared with the unexposed comparison group, new or restarted antidepressant use was associated with a 63% reduced likelihood of predominant, and a 51% reduced likelihood of any breastfeeding at 6 months, as well as a 2.6-fold increased risk of abrupt breastfeeding discontinuation. Specific antidepressants were not mentioned. |
||
| 参考文献 |
Bioorg Med Chem Lett.2008 Feb 15;18(4):1346-9;Psychopharmacology (Berl).2004 Sep;175(2):241-6;Psychopharmacology (Berl).2002 Jul;162(3):323-32.
|
||
| 其他信息 |
Milnacipran hydrochloride is a member of acetamides.
A cyclopropanecarboxamide serotonin and norepinephrine reuptake inhibitor (SNRI) that is used in the treatment of FIBROMYALGIA. See also: Milnacipran (has active moiety). |
| 分子式 |
C15H22N2O.HCL
|
|
|---|---|---|
| 分子量 |
282.81
|
|
| 精确质量 |
282.149
|
|
| CAS号 |
101152-94-7
|
|
| 相关CAS号 |
Milnacipran ((1S-cis) hydrochloride);175131-60-9;Dextromilnacipran;96847-55-1;Milnacipran;92623-85-3;Milnacipran-d5 hydrochloride;2750534-79-1
|
|
| PubChem CID |
163701
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 沸点 |
393ºC at 760 mmHg
|
|
| 熔点 |
179-181ºC
|
|
| 蒸汽压 |
1.66E-07mmHg at 25°C
|
|
| LogP |
3.273
|
|
| tPSA |
46.33
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
2
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
19
|
|
| 分子复杂度/Complexity |
295
|
|
| 定义原子立体中心数目 |
2
|
|
| SMILES |
CCN(CC)C(=O)[C@@]1(C[C@@H]1CN)C2=CC=CC=C2.Cl
|
|
| InChi Key |
XNCDYJFPRPDERF-PBCQUBLHSA-N
|
|
| InChi Code |
InChI=1S/C15H22N2O.ClH/c1-3-17(4-2)14(18)15(10-13(15)11-16)12-8-6-5-7-9-12;/h5-9,13H,3-4,10-11,16H2,1-2H3;1H/t13-,15+;/m1./s1
|
|
| 化学名 |
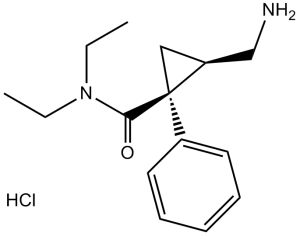
(1R,2S)-2-(aminomethyl)-N,N-diethyl-1-phenylcyclopropane-1-carboxamide hydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.84 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (8.84 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (8.84 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 110 mg/mL (388.95 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.5359 mL | 17.6797 mL | 35.3594 mL | |
| 5 mM | 0.7072 mL | 3.5359 mL | 7.0719 mL | |
| 10 mM | 0.3536 mL | 1.7680 mL | 3.5359 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。