| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
在囊性纤维化 (CF) 折叠上皮 IB3-1 和 CuFi-1 细胞中,miglustat(200 μM;2、4 和 24 小时)可恢复 F508del-CFTR(囊性纤维化跨膜电导调节剂)功能。 Miglustat 可减轻铜绿假单胞菌在 CF 和非 CF 细胞中引起的严重反应 [1]。
|
|---|---|
| 体内研究 (In Vivo) |
Miglustat(0.2 mg/kg;屏障;一次)对过度兴奋做出反应,修复突触可塑性缺陷,并重新激活 ERK [2]。
|
| 动物实验 |
Animal/Disease Models: NPC1−/− mice[1]
Doses: 0.2 mg/kg Route of Administration: po (po (oral gavage)) Experimental Results: Able to rescue synaptic plasticity defects, restore ERK activation and counteract hyperexcitability. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Mean oral bioavailability is 97%. Metabolism / Metabolites There is no evidence that miglustat is metabolized in humans. Biological Half-Life The effective half-life of miglustat is approximately 6 to 7 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In placebo controlled trials, liver test abnormalities were no more common with miglustat than with placebo treatment, and what abnormalities occurred were mild and resolved spontaneously usually without need for dose interruption. During these premarketing clinical trials and since its more widespread clinical availability, no instances of acute liver injury with jaundice have been reported attributable to miglustat. However, the total clinical experience with its use has been limited. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No published experience exists with miglustat during breastfeeding. Because of the lack of information and its side effect profile, most sources consider breastfeeding to be contraindicated during maternal miglustat therapy. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. ◈ What is miglustat? Miglustat is a medication that has been used for treatment of mild to moderate Gaucher disease type 1. It has also been used to treat Niemann-Pick disease type C. Miglustat is sold under the brand name Zavesca®.People with Gaucher disease have low levels of an enzyme called glucocerebrosidase (gloo-co-se-ruh-BRO-si-dace). This enzyme helps break down fatty substances in the body. When this enzyme is missing or not working, fatty substances build up and can cause organ damage. Miglustat works in the body to limit the amount of the fatty substances being made. For more information, see the MotherToBaby fact sheet on Gaucher disease at https://mothertobaby.org/fact-sheets/gaucher-disease-pregnancy/.Sometimes when people find out they are pregnant, they think about changing how they take their medication, or stopping their medication altogether. However, it is important to talk with your healthcare providers before making any changes to how you take this medication. Your healthcare providers can talk with you about the benefits of treating your condition and the risks of untreated illness during pregnancy. ◈ I take miglustat. Can it make it harder for me to get pregnant? Studies have not been done in humans to see if miglustat could make it harder to get pregnant. ◈ Does taking miglustat increase the chance of miscarriage? Miscarriage is common and can occur in any pregnancy for many different reasons. Studies have not been done in humans to see if miglustat could increase the chance for miscarriage. Studies in animals found a greater chance of pregnancy loss at doses around twice as much as would be used in human therapy. ◈ Does taking miglustat increase the chance of birth defects? Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Studies have not been done in humans to see if miglustat increases the chance for birth defects. Animal studies done by the manufacturer did not find an increased chance of births defects. ◈ Does taking miglustat in pregnancy increase the chance of other pregnancy-related problems? Studies have not been done in humans to see if miglustat increases the chance for pregnancy-related problems such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth). Animal studies done by the manufacturer reported a higher chance of low birth weight. ◈ Does taking miglustat in pregnancy affect future behavior or learning for the child? Studies have not been done in humans to see if miglustat causes cause long-term problems in behavior or learning. ◈ Breastfeeding while taking miglustat: There are no studies looking at miglustat use while breastfeeding. The product label for miglustat states that because there is no data available, use during breastfeeding is not recommended. But, the benefit of using miglustat may outweigh possible risks. Your healthcare providers can talk with you about using miglustat and what treatment is best for you. Be sure to talk to your healthcare provider about all of your breastfeeding questions. ◈ If a male takes miglustat, could it affect fertility (ability to get partner pregnant) or increase the chance of birth defects? In humans, one report did not find that miglustat use in 5 males affected the production of sperm or their fertility. Animal studies in rats found that miglustat exposure lowered sperm production, which lowered fertility. However, this was not found in all animal studies; and some animal strains are more sensitive to this exposure. In general, exposures that fathers or sperm donors have are unlikely to increase the risks to a pregnancy. For more information, please see the MotherToBaby fact sheet Paternal Exposures at https://mothertobaby.org/fact-sheets/paternal-exposures-pregnancy/. Protein Binding Miglustat does not bind to plasma proteins. |
| 参考文献 | |
| 其他信息 |
Pharmacodynamics
Miglustat, an N-alkylated imino sugar, is a synthetic analogue of D-glucose. Miglustat is an inhibitor of the enzyme glucosylceramide synthase, which is a glucosyl transferase enzyme responsible for catalyzing the formation of glucosylceramide (glucocerebroside). Glucosylceramide is a substrate for the endogenous glucocerebrosidase, an enzyme that is deficient in Gaucher's disease. The accumulation of glucosylceramide due to the absence of glucocerebrosidase results in the storage of this material in the lysosomes of tissue macrophages, leading to widespread pathology due to infiltration of lipid-engorged macrophages in the viscera, lymph nodes, and bone marrow. This results in secondary hematologic consequences including sever anemia and thrombocytopenia, in addition to the characteristic progressive hepatosplenomegaly, as well as skeletal complications including osteonecrosis and osteopenia with secondary pathological fractures. |
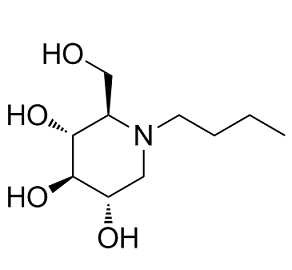
| 分子式 |
C10H21NO4
|
|---|---|
| 分子量 |
219.27804
|
| 精确质量 |
219.147
|
| CAS号 |
72599-27-0
|
| 相关CAS号 |
Miglustat hydrochloride;210110-90-0
|
| PubChem CID |
51634
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
394.7±42.0 °C at 760 mmHg
|
| 熔点 |
169-172 °C
169 - 172 °C |
| 闪点 |
215.4±26.5 °C
|
| 蒸汽压 |
0.0±2.1 mmHg at 25°C
|
| 折射率 |
1.546
|
| LogP |
0.46
|
| tPSA |
84.16
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
15
|
| 分子复杂度/Complexity |
190
|
| 定义原子立体中心数目 |
4
|
| SMILES |
CCCCN1C[C@@H]([C@H]([C@@H]([C@H]1CO)O)O)O
|
| InChi Key |
UQRORFVVSGFNRO-UTINFBMNSA-N
|
| InChi Code |
InChI=1S/C10H21NO4/c1-2-3-4-11-5-8(13)10(15)9(14)7(11)6-12/h7-10,12-15H,2-6H2,1H3/t7-,8+,9-,10-/m1/s1
|
| 化学名 |
(2R,3R,4R,5S)-1-Butyl-2-(hydroxymethyl)piperidine-3,4,5-triol
|
| 别名 |
OGT918 OGT 918 OGT-918 N-butyldeoxynojirimycin NB-DNJ
N-Butylmoranoline Zavesca.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ~250 mg/mL (~1140.09 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.5604 mL | 22.8019 mL | 45.6038 mL | |
| 5 mM | 0.9121 mL | 4.5604 mL | 9.1208 mL | |
| 10 mM | 0.4560 mL | 2.2802 mL | 4.5604 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。