| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| 50g |
|
||
| Other Sizes |
|
| 靶点 |
β1 adrenoceptor
|
|---|---|
| 体外研究 (In Vitro) |
美托洛尔(0-1000 μg/mL;24-72 小时)对 MOLT-4 和 U937 细胞的细胞毒性作用具有剂量和时间依赖性 [3]。
|
| 体内研究 (In Vivo) |
在 ApoE−/− 小鼠中,美托洛尔(2.5 mg/kg/h;输注;11 周)可减少动脉粥样硬化和促炎细胞因子 [1]。美托洛尔(15 mg/kg/q12h;ig;5 天)在由柯萨奇病毒 B3 引起的病毒性心肌炎小鼠模型中显示出抗病毒和抗炎特性 [2]。在患有冠状动脉微栓塞(CME)的大鼠中,美托洛尔(2.5 mg/kg;静脉注射;3次推注)有效防止心肌细胞死亡并减少活化的caspase-9蛋白表达[4]。
|
| 细胞实验 |
细胞毒性测定 [3]
细胞类型: U937 和 MOLT-4 细胞 测试浓度: 1、10、50、100、500 和 1000 μg/ mL 孵育持续时间:24、48 和 72 小时 实验结果:孵育的 U937 和 MOLT -4 细胞的活力显着降低在 1000 μg/mL (3740.14μM) 浓度下孵育 48 小时 (hrs (hours)) 在 ≥500 μg/ml (≥1870.07μM) 浓度下孵育 72 小时 (hrs (hrs) 后,U937 细胞的活力显着降低小时)),并且在孵育 72 小时后,U937 细胞的活力显着降低。 hrs(小时)后,MOLT4细胞浓度≥100 μg/ml(≥374.01μM)。 |
| 动物实验 |
Animal/Disease Models: Male ApoE−/− mice [1]
Doses: 2.5 mg/kg/h Route of Administration: via mini-osmotic pump, 11 weeks Experimental Results: Thoracic aorta atherosclerotic plaque area Dramatically diminished, serum TNFα and chemokine CXCL1, and diminished macrophage content in plaques. Animal/Disease Models: Balb/c mouse, coxsackie virus B3 (CVB3)-induced viral myocarditis (VMC) model [2] Doses: 15 mg/kg/q12h Route of Administration: po (oral gavage), for 5 days Experimental Results: CVB3 infection-induced reduction in VMC pathology score protects myocardium from viral damage by reducing serum cTn-I levels. Reduce myocardial pro-inflammatory cytokine levels and increase anti-inflammatory cytokine expression. Myocardial virus titers were Dramatically diminished. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
When metoprolol is administered orally, it is almost completely absorbed in the gastrointestinal tract. The maximum serum concentration is achieved 20 min after intravenous administration and 1-2 hours after oral administration. The bioavailability of metoprolol is of 100% when administered intravenously and when administered orally it presents about 50% for the tartrate derivative and 40% for the succinate derivative. The absorption of metoprolol in the form of the tartrate derivative is increased by the concomitant administration of food. Metoprolol is mainly excreted via the kidneys. From the eliminated dose, less than 5% is recovered unchanged. The reported volume of distribution of metoprolol is 4.2 L/kg. Due to the characteristics of metoprolol, this molecule is able to cross the blood-brain barrier and even 78% of the administered drug can be found in cerebrospinal fluid. The reported clearance rate on patients with normal kidney function is 0.8 L/min. In cirrhotic patients, the clearance rate changes to 0.61 L/min. Plasma levels following oral administration of conventional metoprolol tablets, however, approximate 50% of levels following intravenous adminsitration, indicating about 50% first-pass metabolism... Elimination is mainly by biotransformation in the liver. Metoprolol tartrate is rapidly and almost completely absorbed from the GI tract; absorption of a single oral dose of 20-100 mg is complete in 2.5-3 hours. After an oral dose, about 50% of the drug administered as conventional tablets appears to undergo first-pass metabolism in the liver. Bioavailability of orally administered metoprolol tartrate increases with increased doses, indicating a possible saturable disposition process of low capacity such as tissue binding in the liver. Steady-state oral bioavailability of extended-release tablets of metoprolol succinate given once daily at dosages equivalent to 50-400 mg of metoprolol tartrate is about 77% of that of conventional tablets at corresponding dosages given once daily or in divided doses. Food does not appear to affect bioavailability of metoprolol succinate extended-release tablets. Following a single oral dose as conventional tablets, metoprolol appears in the plasma within 10 minutes and peak plasma concentrations are reached in about 90 minutes. When metoprolol tartrate conventional tablets are administered with food rather than on an empty stomach, peak plasma concentrations are higher and the extent of absorption of the drug is increased. Following oral administration of metoprolol succinate as extended-release tablets, peak plasma metoprolol concentrations are aobut 25-50% of those attained after administration of metoprolol tartrate conventional tablets given once daily or in divided doses. Time to peak concentration is longer with extended-release tablets, with peak plasma coentrations being reached in about 7 hours following administration of such tablets. Plasma concentrations attained 1 hour after an oral dose are linearly related to metoprolol tartrate doses ranging from 50-400 mg as conventional tablets. Plasma metoprolol concentrations attained after iv administration of the drug are approximately 2 times those attained following oral administration. Following iv infusion of metoprolol over 10 minutes in healthy individuals, maximum beta-adrenergic blocking activity occurred at 20 minutes. In healthy individuals, a maximum reduction in exercise-induced heart rate of approximately 10 and 15% occurs following iv administration of a single 5 mg and 15 mg metoprolol dose, respectively; the effect on exercise-induced heart rate decreased linearly with time at the same rate for both doses and persisted for approximately 5 and 8 hours for the 5 mg and 15 mg doses, respectively. Elimination of metoprolol appears to follow first-order kinetics and occurs mainly in the liver; the time required for the process apparently is independent of dose and duration of therapy. In healthy individuals and hypertensive patients, the elimination half-life of both unchanged drug and metabolites is about 3-4 hours. In poor hydroxylators of the drug, the elimination half-life is prolonged to about 7.6 hours. There is more interindividual variation in elimination half-lives in geriatric patients than in young healthy individuals. The half-life of metoprolol does not increase appreciably with impaired renal function. For more Absorption, Distribution and Excretion (Complete) data for METOPROLOL (7 total), please visit the HSDB record page. Metabolism / Metabolites Metoprolol goes through significant first-pass hepatic metabolism which covers around 50% of the administered dose. The metabolism of metoprolol is mainly driven by the activity of CYP2D6 and to a lesser extent due to the activity of CYP3A4. The metabolism of metoprolol is mainly represented by reactions of hydroxylation and O-demethylation. Metoprolol does not inhibit or enhance its own metabolism. Three main metabolites of the drug are formed by oxidative deamination, O-dealkylation with subsequent oxidation, and aliphatic hydroxylation; these metabolites account for 85% of the total urinary excretion of metabolites. The metabolites apparently do not have appreciable pharmacologic activity. The rate of hydroxylation, resulting in alpha-hydroxymetoprolol, is genetically determined and is subject to considerable interindividual variation. Poor hydroxylators of metoprolol have increased areas under the plasma concentration-time curves, prolonged elimination half-lives (about 7.6 hours), higher urinary concentrations of unchanged drug, and negligible urinary concentrations of alpha-hydroxymetoprolol compared with extensive hydroxylators. Beta-adrenergic blockade of exercise-induced tachycardia persists for at least 24 hours after administration of a single 200-mg oral dose of metoprolol tartrate in poor hydroxylators. Controlled studies have shown that debrisoquine oxidation phenotype is a major determinant of the metabolism, pharmacokinetics and some of the pharmacological actions of metoprolol. The poor metabolizer phenotype is associated with increased plasma drug concentrations, a prolongation of elimination half-life and more intense and sustained beta blockade. Phenotypic differences have also been observed in the pharmacokinetics of the enantiomers of metoprolol. In vivo and in vitro studies have identified some of the metabolic pathways which are subject to the defect, that is alpha-hydroxylation and O-demethylation. Metropolol is a racemic mixture of R-and S-enantiomers, and is primarily metabolized by CYP2D6. Biological Half-Life The immediate release formulations of metoprolol present a half-life of about 3-7 hours. The plasma half-life ranges from approximately 3 to 7 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
The effect of verapamil coadministration on the hepatic first pass clearance of metoprolol was investigated in dogs. Plasma concentration-time course of metoprolol enantiomers and urinary recovery of oxidative metabolites were determined after a single iv (0.51 mg/kg) and an oral (1.37 mg/kg) dose of deuterium labeled pseudoracemic metoprolol, with or without concomitant administration of racemic verapamil (3 mg/kg). Verapamil inhibited both the systemic and oral clearance of metoprolol by about 50-70%. The first pass effect of metoprolol was completely abolished after coadministration of verapamil, reflecting a marked alteration in the degree of hepatic extraction of metoprolol from intermediate to low. The hepatic clearance of metoprolol was slightly (S)-enantioselective (R/S ratio = 0.89 + or - 0.04) in control dogs. Inhibition of hepatic clearance of metoprolol by verapamil was selective towards (S)-metoprolol, such that the enantioselectivity in hepatic clearance toward (S)-metoprolol disappeared following verapamil coadministration (R/S ratio = 1.01 + or - 0.05). Urinary metabolite profiles indicated that O-demethylation and N-dealkylation were the major pathways of oxidative metabolism in the dog. alpha-Hydroxymetoprolol was a minor metabolite in urine. N-Dealkylation showed a strong preference for (S)-metoprolol, whereas O-demethylation and alpha-hydroxylation exhibited a modest selectivity toward (R)-metoprolol; hence, the slight (S)-enantioselectivity in the overall hepatic clearance. Comparison of metoprolol metabolite formation clearances in the absence or presence of verapamil coadministration showed that all three oxidative pathways were inhibited by 60-80%. The greater inhibition of hepatic clearance observed with (S)-metoprolol as compared to (R)-metoprolol was attributed to a significant (S)-enantioselective inhibition in the O-demethylation of metoprolol by verapamil. The interaction between metoprolol and bromazepam and lorazepam was studied in 12 healthy male volunteers aged 21-37 years. Metoprolol had no significant effect on the pharmacokinetics of bromazepam or lorazepam. However, bromazepam area under the curve was 35% higher in the presence of metoprolol. Bromazepam enhanced the effect of metoprolol on systolic blood pressure but not on diastolic blood pressure or pulse rate. Lorazepam had no effect on either blood pressure or pulse. Metoprolol did not enhance the effect of bromazepam on the psychomotor tests used in this study. Metoprolol caused a small increase in critical flicker fusion threshold with lorazepam but had no effect on the other tests. Lorazepam (2 mg) was more potent than bromazepam (6 mg) in the doses used in this study. The interaction of metoprolol with bromazepam and lorazepam is unlikely to be of clinical significance. No change in dose is necessary when using these drugs together. In contrast to early work showing inhibition of the absorption of beta adrenergic blocking drugs by antacids, subsequent studies did not confirm a reduction in the bioavailability of either atenolol or propranolol during antacid treatment; indeed, they showed an increase in the plasma concentrations of metoprolol when the drug was coadministered with an antacid. Caffeine and metoprolol have been reported to increase peak salicylic acid concentration following aspirin administration. For more Interactions (Complete) data for METOPROLOL (12 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat oral 3090-4670 mg/kg LD50 Mice oral 1158-2460 mg/kg LD50 Mouse female iv 118 mg/kg /Metoprolol tartrate/ LD50 Rat male iv ~90 mg/kg /Metoprolol tartrate/ |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Adrenergic beta-Antagonists; Anti-Arrhythmia Agents; Antihypertensive Agents; Sympatholytics Metoprolol /is/ used in the treatment of mitral value prolapse syndrome. /NOT included in US product labeling/ Metoprolol ... /is/ used for thyrotoxicosis. /NOT included in US product labeling/ /Metoprolol has been used/ to control the physical manifestations of anxiety such as tachycardia and tremor. It is not particularly useful for chronic anxiety or panic attacks but is most useful for reducing anxiety and improving performance in specific stressful situations. /NOT included in US product labeling/ For more Therapeutic Uses (Complete) data for METOPROLOL (12 total), please visit the HSDB record page. Drug Warnings Tiredness or dizziness has occurred in about 10% of patients with hypertension or angina receiving metoprolol tartrate in clinical trials; tiredness has been reported in about 1% of patients with myocardial infarction receiving the drug. In addition, vertigo, sleep disturbances/insomnia, hallucinations, nightmares, headache, dizziness, visual disturbances, and confusion have been reported in patients with myocardial infarction receiving the drug, although a causal relationship is unclear. Somnolence or increased dreaming also has been reported with metoprolol therapy; these effects may be alleviated by avoiding late-evening dosing. rarely, impotence, nervousness, and general weakness have occurred. Depression has been reported in about 5% of patients receiving metoprolol tartrate for hypertension or angina. ... /Metoprolol tartrate/ Diarrhea has occurred in about 5% of patients receiving metoprolol tartrate in clinical trials. Other GI symptoms such as nausea, gastric pain, constipation, flatulence, digestive tract disorders, heartburn, xerostomia, and hiccups also have been reported with oral metoprolol therapy. Nausea and abdominal pain have occurred in less than 1% of patients with myocardial infarction receiving IV or oral metoprolol. In 10 healthy subjects administration of metoprolol tartrate 50 mg by mouth increased the peripheral platelet count. Peyronie's disease, tinnitus, restless legs, a polymyalgia-like syndrome, decreased libido, blurred vision, dry eyes, dry mucous membranes, agranulocytosis, and sweating have occurred rarely in patients receiving metoprolol. Pruritus, dry skin, worsening of psoriasis, and psoriasiform, maculopapular, and urticarial rash have occurred in some patients receiving metoprolol. For more Drug Warnings (Complete) data for METOPROLOL (10 total), please visit the HSDB record page. Pharmacodynamics Administration of metoprolol in normal subjects is widely reported to produce a dose-dependent reduction on heart rate and cardiac output. This effect is generated due to a decreased cardiac excitability, cardiac output, and myocardial oxygen demand. In the case of arrhythmias, metoprolol produces its effect by reducing the slope of the pacemaker potential as well as suppressing the rate of atrioventricular conduction. The Metoprolol Atherosclerosis Prevention in Hypertensives (MAPHY) trial showed a significant improvement in sudden cardiac death and myocardial infarction when patients were given with metoprolol as compared with diuretics. As well, in clinical trials performed in 1990, metoprolol reduces mortality and re-infarction in 17% of the individuals when administered chronically after an episode of myocardial infarction. |
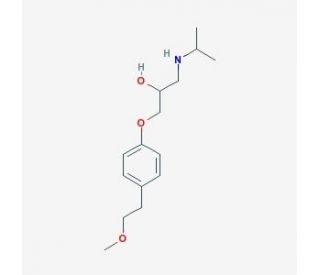
| 分子式 |
C15H25NO3
|
|---|---|
| 分子量 |
267.3639
|
| 精确质量 |
267.183
|
| 元素分析 |
C, 67.38; H, 9.43; N, 5.24; O, 17.95
|
| CAS号 |
51384-51-1
|
| 相关CAS号 |
Metoprolol succinate;98418-47-4;Metoprolol-d7 hydrochloride;1219798-61-4;Metoprolol tartrate;56392-17-7;Metoprolol-d7;959787-96-3;(R)-Metoprolol-d7;1292907-84-6;(S)-Metoprolol-d7;1292906-91-2;Metoprolol-d5;959786-79-9; 51384-51-1; 56392-18-8 (HCl); 80274-67-5 (fumarate); 98418-47-4 (succinate)
|
| PubChem CID |
4171
|
| 外观&性状 |
White to off-white solid
|
| 密度 |
1.0±0.1 g/cm3
|
| 沸点 |
398.6±37.0 °C at 760 mmHg
|
| 闪点 |
194.9±26.5 °C
|
| 蒸汽压 |
0.0±1.0 mmHg at 25°C
|
| 折射率 |
1.508
|
| LogP |
1.79
|
| tPSA |
50.72
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
19
|
| 分子复杂度/Complexity |
215
|
| 定义原子立体中心数目 |
0
|
| SMILES |
OC(CNC(C)C)COC1=CC=C(CCOC)C=C1
|
| InChi Key |
IUBSYMUCCVWXPE-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C15H25NO3/c1-12(2)16-10-14(17)11-19-15-6-4-13(5-7-15)8-9-18-3/h4-7,12,14,16-17H,8-11H2,1-3H3
|
| 化学名 |
1-(isopropylamino)-3-(4-(2-methoxyethyl)phenoxy)propan-2-ol
|
| 别名 |
(RS)-Metoprolol; Beatrolol; dl-Metoprolol; 37350-58-6; Seroken; Spesicor;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~374.03 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (9.35 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (9.35 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7403 mL | 18.7014 mL | 37.4028 mL | |
| 5 mM | 0.7481 mL | 3.7403 mL | 7.4806 mL | |
| 10 mM | 0.3740 mL | 1.8701 mL | 3.7403 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。