| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
Glucocorticoid receptor
|
|---|---|
| 体外研究 (In Vitro) |
HeLa细胞中的胶原酶启动子活性、人PBMC中LPS诱导的IL-12p40以及人PBMC中IFN-γ的植物血凝素水溶液均被丙酮酸甲泼尼龙抑制;这些反应的 IC50 值分别为 9.3、16.8 和 15.2 nM [3]。 MMTV 启动子和 TAT 活性由醋酸甲泼尼龙激活,EC50 值分别为 21.8 和 20.5 nM [3]。
|
| 体内研究 (In Vivo) |
当在 50 μL 巴豆油诱导的埃文蓝水肿模型中局部应用时,醋酸甲泼尼龙丙酯表现出可忽略不计的全身副作用和抗炎特性,IC50 为 0.0015% [1]。局部给药时,醋酸甲泼尼龙 (0.0001%–0.1%) 可减轻小鼠水肿和刺激性接触性皮炎,ED50 为 0.002% [3]。
<人力资源>
在2种炎症动物模型中,局部应用甲基泼尼松龙乙丙酸酯的抗炎效力与很强的糖皮质激素氯倍他索- 17-丙酸相当,但高于17-丁酸氢化可的松。甲基泼尼松龙乙丙酸酯在皮肤中被酶激活。这种激活在发炎组织中进行得更快。与17-丙酸氯倍他索相比,局部应用3天后,甲基泼尼松龙乙丙酸酯没有全身效应。最后,17-丙酸氯倍他索引起明显的皮肤萎缩,而在大鼠皮肤上长期应用(长达43天)后,甲基泼尼松龙乙丙酸酯仅引起轻微的萎缩变化,与17-丁酸氢化可的松的效果相当。
结论:甲基泼尼松龙乙丙酸酯具有高的局部抗炎效力,非常低的全身副作用,只有轻微的局部萎缩活性。局部抗炎作用和萎缩作用分离的原因至今尚不清楚。可以推测,局部抗炎活性非常强的原因之一可能在于炎症组织中的酶活性更快。甲基泼尼松龙乙丙酸酯是一种新的皮质类固醇,它可以改善局部糖皮质激素的抗炎活性和不良副作用之间的分离。[2]
|
| 酶活实验 |
结合和受体选择性[3]
受体结合试验[3] 用重组杆状病毒感染的Sf9细胞提取物,编码人GR、孕激素受体(PR)、雄激素受体(AR)或矿皮质激素受体(MR),进行受体结合试验(Schäcke等人,2004年)。所有受体的命名都遵循“受体和通道指南”(Alexander et al., 2008)。 对于GR, PR, AR和MR [1,2,4,6,7- 3h]地塞米松(约3.18 GBq·mmol - 1, NEN)(约20 nmol·L - 1), [1,2,6,7- 3h (N)]孕酮(约3.7 GBq·mmol - 1, NEN), [17a-甲基- 3h]甲基三烯醇酮(约3.18 GBq·mmol - 1, NEN)或D[1,2,6,7- 3h (N)]醛固酮(约2.81 GBq·mmol - 1, NEN), SF9细胞质(100-500µg蛋白),测试化合物和结合缓冲液(10 mmol·L - 1 Tris/HCL pH 7.4, 1.5 mmol·L - 1 EDTA,10%甘油),以50µL的总体积混合,室温孵育1小时。特异性结合定义为[1,2,4,6,7- 3h]地塞米松、[1,2,6,7- 3h (N)]黄体酮、[17a-甲基- 3h]甲基三烯醇酮和D[1,2,6,7- 3h (N)]醛固酮在10 μ mol·L−1未标记地塞米松、黄体酮、美曲酮或醛固酮不存在和不存在时的结合差异。孵育后,加入50µL冷木炭悬浮液5 min,将混合物转移到微滴过滤板上。将混合物过滤到Picoplates中,并与200µL Microszint-40 混合。结合放射性测定与帕卡德顶级计数平板阅读器。结合曲线的Hill分析确定了抑制50%特异性结合的化合物浓度(IC50)。 选择性测定[3] ZK 245186 SEGRA及其竞争对手化合物在雌激素受体α- (ERα-)、PR-、AR-和mr介导的转激活试验中表现出激动或拮抗活性的潜力被确定。增加测试化合物的浓度:(i)稳定转染了vittk荧光素酶报告基因的MCF-7细胞;(ii)稳定共转染人PR和小鼠乳腺肿瘤病毒(MMTV)荧光素酶报告基因的SK-N-MC细胞;(iii)转染大鼠AR和mmtv -荧光素酶报告基因的CV-1细胞;(iv)转染人MR和mmtv -荧光素酶报告基因的COS-1细胞。测定了受试化合物通过各自的核受体诱导报告基因活性的剂量依赖性,并与参比物雌二醇(ERα)、孕酮(PR)、美曲酮(AR)和醛固酮(MR)的效价和疗效进行了比较。为了检测其拮抗活性,测定了zk245186及其竞争化合物对雌二醇(ERα)、孕酮(PR)、美曲酮(AR)和醛固酮(MR)刺激的报告基因活性的影响。分别与对照化合物氟维司汀(ERα)、米非司酮(PR)、醋酸环丙孕酮(AR)和MR拮抗剂zk91587进行对比。 |
| 细胞实验 |
体外抗炎/免疫调节活性(转抑制)[3]
抑制胶原酶启动子活性[3] 将稳定转染了与胶原酶启动子相关的荧光素酶报告基因的HeLa细胞在Dulbecco改良Eagle培养基中培养24 h,培养基中添加3%炭吸收胎牛血清(FCS)、50单位·mL−1青霉素和50µg·mL−1链霉素、4 mmol·L−1 L-谷氨酰胺和300µg·mL−1遗传素。然后将细胞接种到96孔培养皿中(每孔1 × 104个细胞)。24小时后,用炎症刺激[10 ng·mL−1 12-o-十四烷酰磷酸13-乙酸酯(TPA)]孵育细胞,参比或试验化合物的浓度(1 pmol·L−1至1µmol·L−1)分别升高或不升高。阴性对照细胞(未刺激细胞)用0.1%二甲基亚砜(DMSO)孵育,阳性对照细胞(刺激细胞)用10µg·mL - 1 TPA加0.1% DMSO孵育。18h后进行荧光素酶测定。 受刺激人原代细胞[3] 对细胞因子分泌的抑制作用[3] 所有血细胞的使用均得到献血者的书面同意,符合机构道德准则。用10 ng·mL−1脂多糖(大肠杆菌血清型0127:B8)刺激健康供者外周血单核细胞(PBMCs),测定化合物对IL-12p40单核细胞分泌的影响。用10µg·mL−1的有丝分裂凝集素,植物血凝素刺激PBMC后,检测对干扰素(IFN)-γ分泌的影响。37°C, 5% CO2孵卵24 h后,使用特异性ELISA试剂盒:IFN-γ和IL-12p40 ELISA检测处理细胞上清中细胞因子的浓度。 混合淋巴细胞反应对淋巴细胞增殖的抑制作用[3] 从健康供者获得的人外周血单核细胞在histopque -1077上用肝素化血离心分离,并在添加FCS (10% v/v)的RPMI 1640培养基中培养。对于混合白细胞反应(MLR),将来自一个供体的PBMC与50µg·mL−1丝裂霉素C在37℃下孵育30分钟,然后用PBS反复洗涤,作为刺激细胞。来自非相关供体的PBMCs作为应答细胞,与丝裂霉素c处理的刺激PBMCs (1 × 105细胞/孔)一起接种于96孔圆底微孔板上。培养一式三份,按图2所示的浓度添加化合物,在37°C下孵育。第5天,pbmc用[甲基- 3h]-胸腺嘧啶(每孔7.4 kBq)脉冲标记6小时,然后在玻璃过滤器上收获。[3H]-胸腺嘧啶的掺入用液体闪烁计数法测定,用β -平板闪烁法 定量(z |
| 动物实验 |
Animal/Disease Models: Irritant contact dermatitis in mice and rats [3]: 0.0001%-0.1%
Route of Administration: Topical application, 10 µL for mice and 20 µL for rats Experimental Results: Significant suppression of ear inflammation. Anti-inflammatory activity in vivo [3] Compounds were co-applied with croton oil or with dinitrofluorobenzene (DNFB) in ethanol containing 5% isopropylmyristate or acetone/olive oil 4/1 respectively. Irritant contact dermatitis in mice and rats [3] Both ears of NMRI mice (10 per group) and Wistar rats (10 per group), respectively, were topically treated with compounds or vehicle that were dissolved in croton oil solution (10 µL of 1% for mice and 20 µL of 6.5% for rats). After 24 h, animals were killed and oedema was determined by measuring ear weight in mice or 10 mm diameter ear punch biopsy weight in rats as described earlier (Schäcke et al., 2004). As parameters for neutrophil infiltration, elastase activities were analysed in ear homogenate. The effect of ZK 245186 was compared with methylprednisolone aceponate (MPA) and MF. Each experiment has been repeated twice. Allergic contact dermatitis in mice and rats [3] NMRI mice (10 per treatment group) were sensitized in the skin of the flank with 25 µL of 0.5% DNFB at days 0 and 1. On day 5, mice were challenged by topical application of 20 µL of 0.3% DNFB as described earlier (Zügel et al., 2002). Wistar rats were sensitized with 75 µL of 0.5% DNFB at day 0. On day 5, rats were challenged by topical application of 40 µL 0.4% DNFB. Test or reference compounds (ZK 245186 and methylprednisolone aceponate (MPA) respectively) were topically co-applied in up to four different concentrations (0.001%, 0.01%, 0.1% and 1%) with the hapten challenge. After 24 h, animals were killed to determine ear weight and elastase activity from ear homogenates as parameters for oedema and neutrophil infiltration. Each experiment has been repeated twice. In vivo dissociation and side effect parameters [3] Determination of dissociated in vivo activity in mice as measured by its anti-inflammatory versus pro-diabetogenic effects Determination of the induction of enzymes such as TAT may serve as a suitable surrogate marker for measuring the potential of a compound to act via transactivation in vivo. Two groups of mice (n = 10) were treated on the same day under identical conditions. All compounds were applied s.c., ZK 245186 and methylprednisolone aceponate (MPA) in doses of 1, 3, 10, 30 mg·kg−1 body weight and MF in doses of 0.1, 0.3, 1 mg·kg−1 body weight. One group of mice was used in the croton oil model (main outcome parameter was inhibition of oedema formation) and in the second group of mice, TAT activity was determined from liver homogenates as described earlier (Schäcke et al., 2004). Induction of TAT activity was calculated in relation to vehicle-treated animals as baseline (-fold TAT induction). To determine the therapeutic window or the effect to side effect profile of the compounds, the per cent inhibition of oedema formation on the x-axis was plotted against TAT induction on the y-axis. Glucose tolerance test following topical application of ZK 245186 onto rat skin [3] Hairless rats (hr/hr, n = 10) were treated topically with vehicle, ZK 245186 (0.1%), methylprednisolone aceponate (MPA) (0.1%) or clobetasol propionate (0.1%). Prednisolone (3 mg·kg−1 body weight) p.o. served as a positive control to demonstrate GC-induced changes on the glucose metabolism. Treatment was performed once daily over four consecutive days. On day 5 after an overnight fasting period, 1 g glucose per kg body weight in 10 mL·kg−1 body weight was given orally and blood glucose concentrations were determined immediately before (baseline = 0 min) and shortly afterwards (30, 60, 120, 180 min) using glucose test strips and a blood sugar meter. In house experience with marketed topical GCs showed that compounds that only minimally increase blood glucose concentrations of topically treated hairless rats display low to no risk of altering glucose metabolism in humans after topical use, when applied according to the manufacturer's instructions (H. Wendt et al. unpubl. data). Topical and systemic side effects following prolonged topical application [3] The variables measured were skin fold thickness, skin breaking strength (topical side effects) and total body weight as well as weights of thymus, spleen and the adrenal glands (systemic side effects after topical administration) as described earlier (Schäcke et al., 2004). The treatment regimen was daily application for 3 weeks. Active compounds (ZK 245186, methylprednisolone aceponate (MPA) and MF) or vehicle (EtOH containing 5% isopropylmyristate) were topically applied onto a skin area of 3 cm × 3 cm of juvenile, hr/hr rats (n = 10 per group) in equivalent concentrations for 19 days in a volume of 75 µL. Two different concentrations of the active compounds have been chosen: 3× ED50 determined in the croton oil rat model (for ZK 245186, 0.042%; for methylprednisolone aceponate (MPA), 0.035%; for MF, 0.0027%) and a concentration that results in ca. 80% inhibition of oedema formation in this model (for ZK 245186 and methylprednisolone aceponate (MPA), 0.1% and for MF, 0.01%). Skin fold thickness and animal body weight were determined on days 1 (baseline), 5, 8, 12, 15 and 19. On day 20, animals were killed for determination of the weight of the following organs: thymus, spleen and adrenal glands. The correlation between the skin thinning effects in rodents and humans is well established (Kirby and Munro, 1976; van den Hoven et al., 1991; Woodbury and Kligman, 1992). In this paper, skin atrophy is defined as decrease of skin fold thickness. Body weight, spleen and thymus weight as well as the weight of the adrenal glands are used as measures of undesired systemic effects of topically applied GCs or similar compounds. |
| 毒性/毒理 (Toxicokinetics/TK) |
rat LD50 oral >2 gm/kg GASTROINTESTINAL: HYPERMOTILITY, DIARRHEA; KIDNEY, URETER, AND BLADDER: INCONTINENCE Yakuri to Chiryo. Pharmacology and Therapeutics., 19(3015), 1991
rat LD50 subcutaneous >3 gm/kg ENDOCRINE: OTHER CHANGES Yakuri to Chiryo. Pharmacology and Therapeutics., 19(3015), 1991 |
| 参考文献 |
|
| 其他信息 |
Methylprednisolone aceponate is a corticosteroid hormone.
Methylprednisolone aceponate (MPA) has been shown to provide rapid, reliable and highly effective treatment of eczematous disorders, with an efficacy comparable to that of most reference topical corticosteroids. It also has excellent local and systemic tolerability. MPA is effective in the treatment of facial and scalp eczema and sunburn and has shown promising results in the treatment of psoriasis. Its rapid efficacy and lack of undesirable local and/or systemic side effects make MPA particularly suitable for use in children and infants. The wide range of formulations (0.1%) of MPA, including cream, ointment, fatty ointment, milk and solution, enable treatment to be tailored to the individual patient. In addition, MPA has the advantage of once-daily application compared with twice-daily treatment for other topical corticosteroids, thereby improving patient safety and promoting patient compliance but without compromising efficacy. [1] Background and purpose: Glucocorticoids are highly effective in the therapy of inflammatory diseases. Their value, however, is limited by side effects. The discovery of the molecular mechanisms of the glucocorticoid receptor and the recognition that activation and repression of gene expression could be addressed separately opened the possibility of achieving improved safety profiles by the identification of ligands that predominantly induce repression. Here we report on ZK 245186, a novel, non-steroidal, low-molecular-weight, glucocorticoid receptor-selective agonist for the topical treatment of inflammatory dermatoses. Experimental approach: Pharmacological properties of ZK 245186 and reference compounds were studied in terms of their potential anti-inflammatory and side effects in functional bioassays in vitro and in rodent models in vivo. Key results: Anti-inflammatory activity of ZK 245186 was demonstrated in in vitro assays for inhibition of cytokine secretion and T cell proliferation. In vivo, using irritant contact dermatitis and T cell-mediated contact allergy models in mice and rats, ZK 245186 showed anti-inflammatory efficacy after topical application similar to the classical glucocorticoids, mometasone furoate and methylprednisolone aceponate. ZK 245186, however, exhibits a better safety profile with regard to growth inhibition and induction of skin atrophy after long-term topical application, thymocyte apoptosis, hyperglycaemia and hepatic tyrosine aminotransferase activity. Conclusions and implications: ZK 245186 is a potent anti-inflammatory compound with a lower potential for side effects, compared with classical glucocorticoids. It represents a promising drug candidate and is currently in clinical trials. [3] |
| 分子式 |
C27H36O7
|
|---|---|
| 分子量 |
472.57
|
| 精确质量 |
472.246
|
| 元素分析 |
C, 68.62; H, 7.68; O, 23.70
|
| CAS号 |
86401-95-8
|
| PubChem CID |
63019
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
595.8±50.0 °C at 760 mmHg
|
| 闪点 |
193.1±23.6 °C
|
| 蒸汽压 |
0.0±3.8 mmHg at 25°C
|
| 折射率 |
1.560
|
| LogP |
4.01
|
| tPSA |
106.97
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
34
|
| 分子复杂度/Complexity |
980
|
| 定义原子立体中心数目 |
8
|
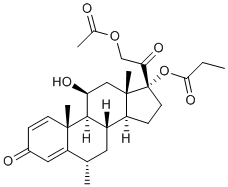
| SMILES |
CCC(=O)O[C@@]1(CC[C@@H]2[C@@]1(C[C@@H]([C@H]3[C@H]2C[C@@H](C4=CC(=O)C=C[C@]34C)C)O)C)C(=O)COC(=O)C
|
| InChi Key |
DALKLAYLIPSCQL-YPYQNWSCSA-N
|
| InChi Code |
InChI=1S/C27H36O7/c1-6-23(32)34-27(22(31)14-33-16(3)28)10-8-19-18-11-15(2)20-12-17(29)7-9-25(20,4)24(18)21(30)13-26(19,27)5/h7,9,12,15,18-19,21,24,30H,6,8,10-11,13-14H2,1-5H3/t15-,18-,19-,21-,24+,25-,26-,27-/m0/s1
|
| 化学名 |
[(6S,8S,9S,10R,11S,13S,14S,17R)-17-(2-acetyloxyacetyl)-11-hydroxy-6,10,13-trimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-yl] propanoate
|
| 别名 |
Advantan; MPA; Methylprednisolone aceponate; 86401-95-8; Advantan; Adventan; Methylprednisolone aceponate [INN]; SH-440; Methylprednisoloni aceponas; Methylprednisoloni aceponas [Latin]; Methylprednisolone Aceponate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1161 mL | 10.5804 mL | 21.1609 mL | |
| 5 mM | 0.4232 mL | 2.1161 mL | 4.2322 mL | |
| 10 mM | 0.2116 mL | 1.0580 mL | 2.1161 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。