| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1g |
|
||
| 5g |
|
||
| Other Sizes |
|
| 靶点 |
Endogenous Metabolite from Microbe and Human
|
|---|---|
| 体外研究 (In Vitro) |
L-赖氨酸水合物 (VSMC) 通过抑制血浆 iPTH 并提高血浆丙氨酸、脯氨酸、血浆精氨酸和高精氨酸来防止细胞凋亡和矿物质沉淀 [1]。
|
| 体内研究 (In Vivo) |
在腺嘌呤大鼠中,口服 40 μg/kg L-赖氨酸水合物可增强血管钙化并保护股骨免受骨质疏松改变[1]。 L-赖氨酸水合物(10 和 400 mg/kg;ig 和 po;雄性小鼠)可抑制胰腺组织损伤 [2]。
血管钙化(VC)是CKD危及生命的并发症。严重的蛋白质限制导致必需氨基酸的缺乏,并加剧了大鼠的VC。因此,我们研究了谷物中第一限制性氨基酸赖氨酸对VC的影响。将13周龄雄性sd大鼠随机分为4组:低蛋白(LP)饲粮(LP组)、LP饲粮+腺嘌呤(Ade组)、LP饲粮+腺嘌呤+甘氨酸(Gly组)作为对照氨基酸组、LP饲粮+腺嘌呤+l-赖氨酸·HCl (Lys组)。18周龄时,LP组无VC,而Ade组和Gly组有相当程度的严重VC。补充赖氨酸几乎完全改善了VC。生理参数和血清肌酐、尿素氮和磷酸盐在Ade组、Gly组和Lys组之间没有差异。值得注意的是,Lys组血清钙略高于Ade组和Gly组,但显著高于Gly组。饲粮赖氨酸能显著抑制腺嘌呤大鼠血浆完整甲状旁腺激素,支持正常的骨血管轴。赖氨酸组股骨磷灰石的保守取向也证明了赖氨酸的骨保护作用。饲粮中赖氨酸升高血浆丙氨酸、脯氨酸、精氨酸和同型精氨酸,但赖氨酸没有升高。体外分析表明,丙氨酸和脯氨酸可抑制培养血管平滑肌细胞的凋亡,精氨酸和同型精氨酸可减弱过饱和钙/磷酸盐溶液中的矿物质沉淀。综上所述,饲粮中添加l-赖氨酸可通过改变导致VC恶化的关键通路来改善VC [1]。 实验分为四组,每组10只。第一组为对照组。II-IV组动物腹腔注射盐酸l -精氨酸(400 mg/kg体重[bw]) 3 d。III组动物口服l -赖氨酸(10 mg/kg bw), IV组动物口服l -赖氨酸(10 mg/kg bw)。血清样品进行淀粉酶、脂肪酶、转氨酶和白细胞介素-6 (IL-6)测定。切除胰腺,测量丙二醛、一氧化氮、过氧化氢酶、超氧化物歧化酶、还原性谷胱甘肽和谷胱甘肽过氧化物酶的水平。 结果:经l -赖氨酸处理前后,丙二醛和一氧化氮水平显著降低,抗氧化酶(超氧化物歧化酶、过氧化氢酶和谷胱甘肽过氧化物酶)和谷胱甘肽活性显著增强(p < 0.001)。然而,作为一种保护剂,赖氨酸的治疗潜力要大于作为一种治疗剂。 结论:l -赖氨酸处理可通过抑制炎性细胞因子IL-6的释放,增强抗氧化活性,减轻l -精氨酸诱导的胰腺组织损伤。这些影响可能涉及抗炎因子的上调和随后IL6的下调。[2] |
| 动物实验 |
Animal/Disease Models: Male mice [2]
Doses: 10 and 400 mg/kg Route of Administration: intraperitoneal (ip) injection and oral administration; 15 days Experimental Results: Inhibited the release of inflammatory cytokine IL-6 and enhanced antioxidant activity. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorption Absorbed from the lumen of the small intestine into the enterocytes by an active transport process Although the free amino acids dissolved in the body fluids are only a very small proportion of the body's total mass of amino acids, they are very important for the nutritional and metabolic control of the body's proteins. ... Although the plasma compartment is most easily sampled, the concentration of most amino acids is higher in tissue intracellular pools. Typically, large neutral amino acids, such as leucine and phenylalanine, are essentially in equilibrium with the plasma. Others, notably glutamine, glutamic acid, and glycine, are 10- to 50-fold more concentrated in the intracellular pool. Dietary variations or pathological conditions can result in substantial changes in the concentrations of the individual free amino acids in both the plasma and tissue pools. /Amino acids/ After ingestion, proteins are denatured by the acid in the stomach, where they are also cleaved into smaller peptides by the enzyme pepsin, which is activated by the increase in stomach acidity that occurs on feeding. The proteins and peptides then pass into the small intestine, where the peptide bonds are hydrolyzed by a variety of enzymes. These bond-specific enzymes originate in the pancreas and include trypsin, chymotrypsins, elastase, and carboxypeptidases. The resultant mixture of free amino acids and small peptides is then transported into the mucosal cells by a number of carrier systems for specific amino acids and for di- and tri-peptides, each specific for a limited range of peptide substrates. After intracellular hydrolysis of the absorbed peptides, the free amino acids are then secreted into the portal blood by other specific carrier systems in the mucosal cell or are further metabolized within the cell itself. Absorbed amino acids pass into the liver, where a portion of the amino acids are taken up and used; the remainder pass through into the systemic circulation and are utilized by the peripheral tissues. /Amino acids/ Protein secretion into the intestine continues even under conditions of protein-free feeding, and fecal nitrogen losses (ie, nitrogen lost as bacteria in the feces) may account for 25% of the obligatory loss of nitrogen. Under this dietary circumstance, the amino acids secreted into the intestine as components of proteolytic enzymes and from sloughed mucosal cells are the only sources of amino acids for the maintenance of the intestinal bacterial biomass. ... Other routes of loss of intact amino acids are via the urine and through skin and hair loss. These losses are small by comparison with those described above, but nonetheless may have a significant impact on estimates of requirements, especially in disease states. /Amino acids/ About 11 to 15 g of nitrogen are excreted each day in the urine of a healthy adult consuming 70 to 100 g of protein, mostly in the form of urea, with smaller contributions from ammonia, uric acid, creatinine, and some free amino acids. These are the end products of protein metabolism, with urea and ammonia arising from the partial oxidation of amino acids. Uric acid and creatinine are indirectly derived from amino acids as well. The removal of nitrogen from the individual amino acids and its conversion to a form that can be excreted by the kidney can be considered as a two-part process. The first step usually takes place by one of two types of enzymatic reactions: transamination or deamination. Transamination is a reversible reaction that uses ketoacid intermediates of glucose metabolism (e.g., pyruvate, oxaloacetate, and alpha-ketoglutarate) as recipients of the amino nitrogen. Most amino acids can take part in these reactions, with the result that their amino nitrogen is transferred to just three amino acids: alanine from pyruvate, aspartate from oxaloacetate, and glutamate from alpha-ketoglutarate. Unlike many amino acids, branched-chain amino acid transamination occurs throughout the body, particularly in skeletal muscle. Here the main recipients of amino nitrogen are alanine and glutamine (from pyruvate and glutamate, respectively), which then pass into the circulation. These serve as important carriers of nitrogen from the periphery (skeletal muscle) to the intestine and liver. In the small intestine, glutamine is extracted and metabolized to ammonia, alanine, and citrulline, which are then conveyed to the liver via the portal circulation. Nitrogen is also removed from amino acids by deamination reactions, which result in the formation of ammonia. A number of amino acids can be deaminated, either directly (histidine), by dehydration (serine, threonine), by way of the purine nucleotide cycle (aspartate), or by oxidative deamination (glutamate). ... Glutamate is also formed in the specific degradation pathways of arginine and lysine. Thus, nitrogen from any amino acid can be funneled into the two precursors of urea synthesis, ammonia and aspartate. /Amino acids/ Metabolism / Metabolites Hepatic Like other amino acids, the metabolism of free lysine follows two principal paths: protein synthesis and oxidative catabolism. It is required for biosynthesis of such substances as carnitine, collage, and elastin. Oxidative deamination or transamination of l-lysine /yields/ alpha-keto-epsilon-aminocaproic acid; decarboxylation of l-lysine /yields/ cadaverine. /From table/ Once the amino acid deamination products enter the tricarboxylic acid (TCA) cycle (also known as the citric acid cycle or Krebs cycle) or the glycolytic pathway, their carbon skeletons are also available for use in biosynthetic pathways, particularly for glucose and fat. Whether glucose or fat is formed from the carbon skeleton of an amino acid depends on its point of entry into these two pathways. If they enter as acetyl-CoA, then only fat or ketone bodies can be formed. The carbon skeletons of other amino acids can, however, enter the pathways in such a way that their carbons can be used for gluconeogenesis. This is the basis for the classical nutritional description of amino acids as either ketogenic or glucogenic (ie, able to give rise to either ketones [or fat] or glucose). Some amino acids produce both products upon degradation and so are considered both ketogenic and glucogenic. /Amino acids/ ... Rates of lysine metabolism in fetal sheep during chronic hypoglycemia and following euglycemic recovery /were compared with/ results with normal, age-matched euglycemic control fetuses to explain the adaptive response of protein metabolism to low glucose concentrations. Restriction of the maternal glucose supply to the fetus lowered the net rates of fetal (umbilical) glucose (42%) and lactate (36%) uptake, causing compensatory alterations in fetal lysine metabolism. The plasma lysine concentration was 1.9-fold greater in hypoglycemic compared with control fetuses, but the rate of fetal (umbilical) lysine uptake was not different. In the hypoglycemic fetuses, the lysine disposal rate also was higher than in control fetuses due to greater rates of lysine flux back into the placenta and into fetal tissue. The rate of CO2 excretion from lysine decarboxylation was 2.4-fold higher in hypoglycemic than control fetuses, indicating greater rates of lysine oxidative metabolism during chronic hypoglycemia. No differences were detected for rates of fetal protein accretion or synthesis between hypoglycemic and control groups, although there was a significant increase in the rate of protein breakdown (p < 0.05) in the hypoglycemic fetuses, indicating small changes in each rate. This was supported by elevated muscle specific ubiquitin ligases and greater concentrations of 4E-BP1. Euglycemic recovery after chronic hypoglycemia normalized all fluxes and actually lowered the rate of lysine decarboxylation compared with control fetuses (p < 0.05). These results indicate that chronic hypoglycemia increases net protein breakdown and lysine oxidative metabolism, both of which contribute to slower rates of fetal growth over time. Furthermore, euglycemic correction for 5 days returns lysine fluxes to normal and causes an overcorrection of lysine oxidation. |
| 毒性/毒理 (Toxicokinetics/TK) |
rat LD50 oral 11400 mg/kg Gekkan Yakuji. Pharmaceuticals Monthly., 23(1253), 1981 rat LD50 intraperitoneal 3700 mg/kg Gekkan Yakuji. Pharmaceuticals Monthly., 23(1253), 1981 rat LD50 subcutaneous 4 gm/kg Iyakuhin Kenkyu. Study of Medical Supplies., 12(933), 1981 rat LD50 intravenous 2850 mg/kg Gekkan Yakuji. Pharmaceuticals Monthly., 23(1253), 1981 mouse LD50 oral 13400 mg/kg Gekkan Yakuji. Pharmaceuticals Monthly., 23(1253), 1981 Toxicity Summary Proteins of the herpes simplex virus are rich in L-arginine, and tissue culture studies indicate an enhancing effect on viral replication when the amino acid ratio of L-arginine to L-lysine is high in the tissue culture media. When the ratio of L-lysine to L-arginine is high, viral replication and the cytopathogenicity of herpes simplex virus have been found to be inhibited. L-lysine may facilitate the absorption of calcium from the small intestine. Health Effects Chronically high levels of lysine are associated with at least 5 inborn errors of metabolism including: D-2-Hydroxyglutaric Aciduria, Familial Hyperlysinemia I, Hyperlysinemia II, Pyruvate carboxylase deficiency and Saccharopinuria. Exposure Routes Absorbed from the lumen of the small intestine into the enterocytes by an active transport process Interactions Lysine 10 mmol/kq given to mice for 1 to 10 days significantly increased clonic and tonic seizure latencies caused by 60 mg/kg pentylenetetrazol (PTZ). On day 1 the clonic and tonic seizure latencies were increased from 160.4 +/- 26.3 and 828.6 +/- 230.8 s to 286.1 +/- 103.3 and 982.3 +/- 98.6 respectively. Both clonic and tonic seizure latencies increased steadily with additional L-lysine treatment without significant change in survival rate. On day 10, the anticonvulsant effect reached its highest level with a block of tonic seizures and survival rate of 100% without tolerance developing. Acute L-lysine significantly increased the mean clonic latency from 85.8 +/- 5.24 to 128.2 +/- 9.0 s and the mean tonic seizure from 287.2 +/- 58.7 to 313.5 +/- 42.2 s with 80 mg/kg of PTZ. On day 10 of treatment, the anticonvulsant effect of L-lysine was highest, with a significant incr of 155 and 184% in clonic and tonic latencies over control, respectively. After 15 and 20 day treatment, clonic and tonic seizure latencies and survival rate decreased, suggesting development of tolerance ... PMID:8385623 Acute intake of high levels of lysine interferes with dietary protein metabolism and competes with the transport of arginine, suggesting that adverse effects from high levels of lysine are more likely to occur if protein intake or dietary arginine intake is low. |
| 参考文献 | |
| 其他信息 |
L-Lysine is a nutritional supplement containing the biologically active L-isomer of the essential amino acid lysine, with potential anti-mucositis activity. Upon oral intake, L-lysine promotes healthy tissue function, growth and healing and improves the immune system. L-Lysine promotes calcium uptake, is essential for carnitine production and collagen formation. As collagen is essential for connective tissue maintenance, this agent may also help heal mucosal wounds. This may help decrease and prevent mucositis induced by radiation or chemotherapy.
See also: Lysine (annotation moved to). |
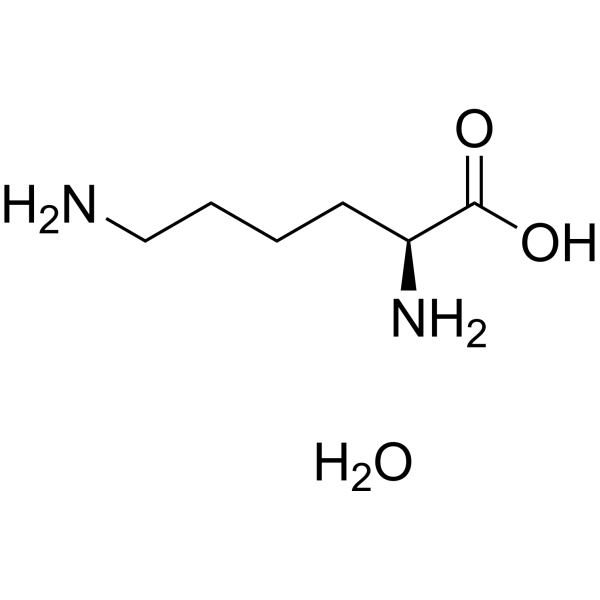
| 分子式 |
C6H16N2O3
|
|---|---|
| 分子量 |
164.2028
|
| 精确质量 |
164.116
|
| 元素分析 |
C, 43.89; H, 9.82; N, 17.06; O, 29.23
|
| CAS号 |
39665-12-8
|
| 相关CAS号 |
L-Lysine;56-87-1;L-Lysine hydrochloride;657-27-2;L-Lysine acetate;57282-49-2
|
| PubChem CID |
16211825
|
| 外观&性状 |
White to yellow solid powder
|
| 沸点 |
400ºC at 760 mmHg
|
| 熔点 |
212-214 °C
|
| 闪点 |
195.7ºC
|
| LogP |
0.863
|
| tPSA |
98.57
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
11
|
| 分子复杂度/Complexity |
106
|
| 定义原子立体中心数目 |
1
|
| SMILES |
C(CCN)C[C@@H](C(=O)O)N.O
|
| InChi Key |
HZRUTVAFDWTKGD-JEDNCBNOSA-N
|
| InChi Code |
InChI=1S/C6H14N2O2.H2O/c7-4-2-1-3-5(8)6(9)10;/h5H,1-4,7-8H2,(H,9,10);1H2/t5-;/m0./s1
|
| 化学名 |
(2S)-2,6-diaminohexanoic acid;hydrate
|
| 别名 |
L-Lysine monohydrate; 39665-12-8; L-Lysine hydrate; Lysine monohydrate; L-Lysine, hydrate; l-Lysine, monohydrate; 199926-21-1; L(+)-Lysine monohydrate;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 6.0901 mL | 30.4507 mL | 60.9013 mL | |
| 5 mM | 1.2180 mL | 6.0901 mL | 12.1803 mL | |
| 10 mM | 0.6090 mL | 3.0451 mL | 6.0901 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。