| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| 50g |
|
||
| 100g |
|
||
| Other Sizes |
|

| 靶点 |
TOPO IV
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Bioavailability of ofloxacin in the tablet formulation is approximately 98% Ofloxacin is mainly eliminated by renal excretion, where between 65% and 80% of an administered oral dose of ofloxacin is excreted unchanged via urine within 48 hours of dosing. About 4-8% of an ofloxacin dose is excreted in the feces and the drug is minimally subject to biliary excretion. Ofloxacin is distributed into bone, cartilage, bile, skin, sputum, bronchial secretions, pleural effusions, tonsils, saliva, gingival mucosa, nasal secretions, aqueous humor, tears, sweat, lung, blister fluid, pancreatic fluid, ascitic fluid, peritoneal fluid, gynecologic tissue, vaginal fluid, cervix, ovary, semen, prostatic fluid, and prostatic tissue. For most of these tissues and fluids, ofloxacin concentrations are approximately 0.5-1.7 times concurrent serum concentrations. Ofloxacin is concentrated within neutrophils, achieving concentrations in these cells that may be up to 8 times greater than extracellular concentrations. Ofloxacin is widely distributed into body tissues and fluids following oral administration. In healthy adults, the apparent volume of distribution of ofloxacin averages 1-2.5 L/kg. Impaired renal function does not appear to affect the volume of distribution of ofloxacin; the apparent volume of distribution of the drug averages 1.1-2 L/kg in patients with impaired renal function, including those with severe renal failure undergoing hemodialysis. Pharmacokinetic parameters in geriatric patients receiving ofloxacin generally are similar to those in younger adults. Although results of pharmacokinetic studies in geriatric individuals 65-81 years of age indicate that the rate of absorption, volume of distribution, and route of excretion in geriatric individuals are similar to those in younger adults, peak serum concentrations are slightly higher (9-21% higher) and half-life more prolonged in geriatric patients than in younger adults. There also is evidence that peak plasma concentration are higher in geriatric women than geriatric men (114% higher following single doses or 54% higher following multiple doses). The oral bioavailability of ofloxacin is 85-100% in healthy, fasting adults, and peak serum concentrations of the drug generally are attained within 0.5-2 hours. In patients with normal renal and hepatic function, peak serum concentrations and AUCs increase in proportion to the dose over the oral dosage range of 100-600 mg and generally are unaffected by age. Following oral administration of a single 100-, 200-, 300-, or 400-mg dose of ofloxacin in healthy, fasting adults, peak serum concentrations average 1-1.3, 1.5-2.7, 2.4-4.6, or 2.9-5.6 ug/mL, respectively. Some accumulation occurs following multiple doses. Steady-state serum concentrations of ofloxacin are achieved after 4 doses of the drug and are approximately 40% higher than concentrations achieved following single oral doses. For more Absorption, Distribution and Excretion (Complete) data for Ofloxacin (18 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic Less than 10% of a single dose of ofloxacin is metabolized; approximately 3-6% of the dose is metabolized to desmethyl ofloxacin and 1-5% is metabolized to ofloxacin N-oxide. Desmethyl ofloxacin is microbiologically active, but is less active against susceptible organisms than is ofloxacin; ofloxacin N-oxide has only minimal antibacterial activity. Seven patients with end-stage renal disease on regular hemodialysis were treated orally with a loading dose of 200 mg ofloxacin and multiple maintenance doses of 100 mg per 24 hr for 10 days. The pharmacokinetics of ofloxacin and its metabolites were studied at the end of the treatment period. Plasma and dialysate concentrations of ofloxacin and ofloxacin metabolites were measured by HPLC. Peak (3.1 mg.L-1) and trough levels (1.6 mg.L-1) and the AUC of ofloxacin were comparable to the values in healthy volunteers given 300 to 400 mg ofloxacin p.o. The mean half-life, determined in the dialysis-free interval (t1/2 beta) and during the haemodialysis session (t1/2 HD), was 38.5 h and 9.9 h, respectively. Extrarenal clearance (32.7 mL.min-1) was unchanged as compared to that reported in healthy volunteers after a single dose of ofloxacin. The fractional removal by haemodialysis amounted to 21.5%. Two metabolites, ofloxacin-N-oxide and demethyl-ofloxacin, were detected in plasma. Despite prolonged t1/2 beta of both metabolites (66.1 and 50.9 hr) and multiple doses of ofloxacin the peak concentrations of the metabolites reached only 14% and 5% of that of the parent drug, respectively. It is concluded that in patients on regular hemodialysis treatment the dosage adjustment employed resulted in safe and therapeutically favourable plasma concentrations. The observed accumulation of ofloxacin metabolites does not appear to have any toxic or therapeutic significance. Biological Half-Life 9 hours In adults with creatinine clearances of 10-50 mL/minute, half-life of the drug averages 16.4 hours (range: 11-33.5 hours); in adults with creatinine clearances less than 10 mL/minute, half-life averages 21.7 hours (range: 16.9-28.4 hours). In patients with end-stage renal failure, half-life of the drug may range from 25-48 hours. In healthy adults with normal renal function, the elimination half-life of ofloxacin in the distribution phase averages 0.5-0.6 hours and the elimination half-life in the terminal phase averages 4-8 hours.In healthy geriatric adults 64-86 years of age with renal function normal for their age, half-life of the drug averages 6.4-8.5 hours. Following ocular instillation of 1 drop of ofloxacin 0.3% 4 times daily for 12 doses in healthy individuals, the elimination half-life of drug in tear film was approximately 226 minutes. In a study in rabbits, the terminal elimination half-life of ofloxacin in tear film following topical application to the eye was approximately 210 minutes. In adults with normal renal function, the serum elimination half-life of ofloxacin in the terminal phase averages 4-8 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Mild elevations in ALT and alkaline phosphatase levels occur in 1 to 2% of patients on ofloxacin. These abnormalities are generally mild, asymptomatic and transient, resolving even with continuation of therapy. Ofloxacin has also been linked to rare but occasionally severe and even fatal cases of acute liver injury. The time to onset is typically short (2 days to 2 weeks) and the presentation is often abrupt with nausea, fatigue, abdominal pain and jaundice. The pattern of serum enzyme elevations can be either hepatocellular or cholestatic, cases with the shorter times to onset usually being more hepatocellular with markedly elevated ALT levels, and occasionally with rapid worsening of prothrombin time and signs of hepatic failure. The onset of illness may occur a few days after the medication is stopped. Cases with a cholestatic pattern of enzymes may run a prolonged course but are usually self-limiting. Many (but not all) cases have had allergic manifestations with fever, rash and eosinophilia. Autoantibodies are usually not present. The hepatotoxicity of ofloxacin is similar to that of other fluoroquinolones and appears to represent a class effect. Likelihood score: A (well established but rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Ofloxacin appears in breastmilk in low levels. Fluoroquinolones have traditionally not been used in infants because of concern about adverse effects on the infants' developing joints. However, recent studies indicate little risk. The calcium in milk might prevent absorption of the small amounts of fluoroquinolones in milk. Insufficient data exist to prove or disprove this assertion. Developmental problems have been reported in two infants exposed to ofloxacin in breastmilk, but their mothers were also exposed to several drugs during pregnancy and during breastfeeding, so the problems cannot necessarily be attributed to ofloxacin. Use of ofloxacin is acceptable in nursing mothers with monitoring of the infant for possible effects on the flora, such as diarrhea or candidiasis (thrush, diaper rash). . Avoiding breastfeeding for 4 to 6 hours after a dose should decrease the exposure of the infant to ofloxacin in breastmilk. Maternal use of an ear drop or eye drop that contains ofloxacin presents negligible risk for the nursing infant. To substantially diminish the amount of drug that reaches the breastmilk after using eye drops, place pressure over the tear duct by the corner of the eye for 1 minute or more, then remove the excess solution with an absorbent tissue. ◉ Effects in Breastfed Infants Ofloxacin was used as part of multidrug regimens to treat two pregnant women with multidrug-resistant tuberculosis, one throughout pregnancy and postpartum and the other postpartum only. The infants were breastfed (extent and duration not stated). At age 4.6 and 5.1 years, the children were developing normally except for a mild speech delay in one and hyperactivity in the other. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding 32% Interactions Concomitant administration of some fluoroquinolone anti-infectives (e.g., ciprofloxacin, norfloxacin, ofloxacin) in patients receiving theophylline has resulted in higher and prolonged serum theophylline concentrations and may increase the risk of theophylline-related adverse effects. The extent of this interaction varies considerably among the commercially available fluoroquinolones; the effect is less pronounced with norfloxacin or ofloxacin than with ciprofloxacin. While it has been suggested that the 4-oxo metabolites of these quinolones may inhibit metabolism of theophylline in the liver, and there is some evidence that the degree to which the various quinolones are metabolized to 4-oxo metabolites may correlate with the extent of alteration in theophylline pharmacokinetics when the drugs are administered concomitantly, the potential contribution, if any, of the 4-oxo metabolites to this interaction has not been fully elucidated. In addition, other evidence indicates that, while formation of these metabolites may correlate with inhibition of theophylline metabolism, the 4-oxo metabolites themselves are not responsible for the observed effect. Studies using other fluoroquinolones (e.g., ciprofloxacin) indicate that concomitant administration of probenecid interferes with renal tubular secretion of the drugs. The effect of concomitant administration of probenecid and ofloxacin has not been studied to date. Concomitant administration of a fluoroquinolone (i.e., ofloxacin) and fenbufen (a nonsteroidal anti-inflammatory agent (NSAIA)) reportedly resulted in an increased incidence of seizures. Concomitant use of a fluoroquinolone with an NSAIA could increase the risk of CNS stimulation (e.g., seizures). Animal studies using other fluoroquinolones suggest that the risk may vary depending on the specific NSAIA. Oral multivitamin and mineral supplements containing divalent or trivalent cations such as iron or zinc may decrease oral absorption of ofloxacin resulting in decreased serum concentrations of the quinolone; therefore, these multivitamins and/or mineral supplements should not be ingested concomitantly with or within 2 hours of an ofloxacin dose. In a crossover study, concomitant administration of a single dose of oral ferrous sulfate complex and ofloxacin decreased the AUC of the anti-infective by 36%. For more Interactions (Complete) data for Ofloxacin (19 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat iv 273 mg/kg LD50 Rat sc 7070 mg/kg LD50 Rat oral 3590 mg/kg LD50 Monkey oral 500 mg/kg For more Non-Human Toxicity Values (Complete) data for Ofloxacin (6 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Anti-Bacterial Agents; Anti-Infective Agents, Urinary; Nucleic Acid Synthesis Inhibitors Ofloxacin is used in the treatment of acute pelvic inflammatory disease (PID) caused by susceptible C. trachomatis or N. gonorrhoeae, but should not be used if QRNG may be involved or if in vitro susceptibility cannot be tested. /Included in US product label/ Ofloxacin is used in adults for the treatment of nongonococcal urethritis and cervicitis caused by Chlamydia trachomatis. /Included in US product label/ Ofloxacin is used in adults for the treatment of uncomplicated urinary tract infections (UTIs) (cystitis) caused by susceptible gram-negative bacteria, including Citrobacter diversus, ... Enterobacter aerogenes, ... Escherichia coli, Klebsiella pneumoniae, ... Proteus mirabilis, or Pseudomonas aeruginosa. /Included in US product label/ For more Therapeutic Uses (Complete) data for Ofloxacin (36 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ WARNING: Fluoroquinolones, including ofloxacin, are associated with an increased risk of tendinitis and tendon rupture in all ages. This risk is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants. /BOXED WARNING/ WARNING: Fluoroquinolones, including ofloxacin, may exacerbate muscle weakness in persons with myasthenia gravis. Avoid ofloxacin in patients with known history of myasthenia gravis. Some quinolones, including ofloxacin, have been associated with prolongation of the QT interval on the electrocardiogram and infrequent cases of arrhythmia. Rare cases of torsade de pointes have been spontaneously reported during postmarketing surveillance in patients receiving quinolones, including ofloxacin. Rare cases of sensory or sensorimotor axonal polyneuropathy affecting small and/or large axons resulting in paresthesias, hypoesthesias, dysesthesias and weakness have been reported in patients receiving quinolones, including ofloxacin. Ofloxacin should be discontinued if the patient experiences symptoms of neuropathy including pain, burning, tingling, numbness, and/or weakness or other alterations of sensation including light touch, pain, temperature, position sense, and vibratory sensation in order to prevent the development of an irreversible condition. For more Drug Warnings (Complete) data for Ofloxacin (28 total), please visit the HSDB record page. Pharmacodynamics Ofloxacin is a quinolone/fluoroquinolone antibiotic. Ofloxacin is bactericidal and its mode of action depends on blocking of bacterial DNA replication by binding itself to an enzyme called DNA gyrase, which allows the untwisting required to replicate one DNA double helix into two. Notably the drug has 100 times higher affinity for bacterial DNA gyrase than for mammalian. Ofloxacin is a broad-spectrum antibiotic that is active against both Gram-positive and Gram-negative bacteria. |
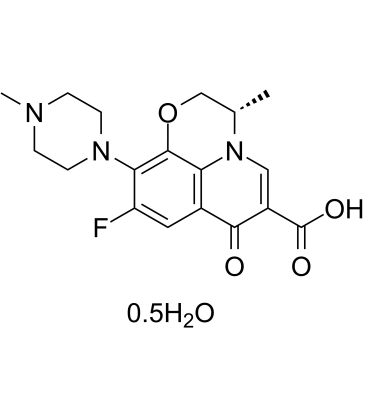
| 分子式 |
C36H42F2N6O9
|
|---|---|
| 分子量 |
740.76
|
| 精确质量 |
740.2981
|
| 元素分析 |
C, 58.37; H, 5.72; F, 5.13; N, 11.35; O, 19.44
|
| CAS号 |
138199-71-0
|
| 相关CAS号 |
Levofloxacin;100986-85-4;Levofloxacin hydrochloride;177325-13-2;(R)-Ofloxacin;100986-86-5
|
| PubChem CID |
4583
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.48g/cm3
|
| 沸点 |
571.5ºC at 760mmHg
|
| 熔点 |
214-216°C
|
| 蒸汽压 |
6.7E-14mmHg at 25°C
|
| LogP |
1.482
|
| tPSA |
84.24
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
26
|
| 分子复杂度/Complexity |
634
|
| 定义原子立体中心数目 |
0
|
| SMILES |
FC1C([H])=C2C(C(C(=O)O[H])=C([H])N3C2=C(C=1N1C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])C1([H])[H])OC([H])([H])C3([H])C([H])([H])[H])=O
|
| InChi Key |
SUIQUYDRLGGZOL-RCWTXCDDSA-N
|
| InChi Code |
InChI=1S/2C18H20FN3O4.H2O/c2*1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21;/h2*7-8,10H,3-6,9H2,1-2H3,(H,24,25);1H2/t2*10-;/m00./s1
|
| 化学名 |
(S)-9-Fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid hemihydrate
|
| 别名 |
RWJ 25213; RWJ-25213; RWJ25213; Levofloxacin Hydrate; Levofloxacin Hemihydrate
|
| HS Tariff Code |
2934.99.03.00
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ≥ 50 mg/mL (~135.00 mM)
Ethanol : ~10 mg/mL DMSO : ~8.33 mg/mL (~22.49 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 0.83 mg/mL (2.24 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 8.3 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 0.83 mg/mL (2.24 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 8.3 mg/mL 澄清 DMSO 储备液加入 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 0.83 mg/mL (2.24 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 0.83 mg/mL (2.24 mM) 配方 5 中的溶解度: 100 mg/mL (269.99 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3500 mL | 6.7498 mL | 13.4996 mL | |
| 5 mM | 0.2700 mL | 1.3500 mL | 2.6999 mL | |
| 10 mM | 0.1350 mL | 0.6750 mL | 1.3500 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Diagnostics of Chronic Endometritis in Infertility
CTID: NCT05946655
Phase: N/A Status: Completed
Date: 2023-11-18