| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Leuprolide is typically administered as a single-dose long-acting formulation employing either microsphere or biodegradable solid depot technologies. Regardless of the exact formulation and initial dose strength, the Cmax is typically achieved by 4-5 hours post-injection and displays large variability in the range of 4.6 - 212 ng/mL. Eventual steady-state kinetics are typically achieved by four weeks, with a narrower range of 0.1 - 2 ng/mL. No studies on the effects of food on absorption have been carried out. Following administration of 3.75 mg leuprolide depot suspension to three patients, less than 5% of the initial dose was recovered as unchanged or pentapeptide metabolite in the urine. Leuprolide has an apparent steady-state volume of distribution of 27 L following intravenous bolus administration to healthy males. The volume of distribution for indicated routes of subcutaneous or intramuscular injection has not been reported. Leuprolide administered as a 1 mg intravenous bolus in healthy males has a mean systemic clearance between 7.6 and 8.3 L/h. Bioavailablity after intramuscular injection of the depot formulation is estimated to be about 90%. The pharmacological effects of leuprolide acetate depot microspheres were studied in rats and dogs following subcutaneous and intramuscular injection. After injection the microspheres provided similar linear drug release and sustained serum drug levels for 3 months. Persistent suppression of serum luteinizing hormone, follicle stimulating hormone in rats, and testosterone in rats and dogs for over 16 wk was achieved with microspheres at a dose of 100 ug/kg/day in rats and 25.6 ug/kg/day in dogs. Responses upon periodic challenge tests revealed that a single injection of microspheres dramatically suppressed the function of the pituitary-gonadal system for 15 wks in rats. The growth of genital organs was also suppressed dose-dependently for over 3 months. It was concluded that persistent pharmacological effects are obtained with an injection of leuprolide 3-month depot microspheres. The effect of formulation adjuvants on the absorption of leuprolide acetate after intraduodenal injection and oral administration to male castrate rats is reported. Absorption was low, approximately 0.01% and 0.08% by oral and intraduodenal administration, respectively, compared with intravenous controls. An aqueous formulation and a water-in-oil emulsion of a lipophilic salt, a decane sulfonic acid derivative of leuprolide gave intraduodenal bioavailabilities of approximately 0.2% and 1% respectively. Evaluation of formulation effects on the oral absorption of the drug showed that lipophilicity, surfactant, and vehicle properties significantly affected intraduodenal absorption of leuprolide. Absolute bioavailability of the drug in typical emulsion systems ranged from approximately 3-10% and represented an improvement of about 100-fold in gastrointestinal bioavailability of this peptide. The implications of these findings relative to the effect of formulation adjuvants on oral absorption of leuprolide and other peptides following intraduodenal administration are discussed. The bioavailability of leuprolide acetate was studied in rats and in healthy males (ages 19-39 yr) after inhalation and intranasal administration, compared with intravenous and subcutaneous injection. Intranasal bioavailability in rats was significantly increased by alpha-cyclodextrin, eidetic acid, and solution volume. Intra-animal variability was 30-60% and absorption ranged from 8 to 46% compared with intravenous controls. In humans, the subcutaneous injection was 94% bioavailable compared with intravenous. Intranasal bioavailability averaged 2.4%, with significant intersubject variability. Plasma peak concentrations for one and 3 mg dosages were 0.24-1.6 and 0.1-11 ng/ml, respectively. Mean plasma peak concentrations of one mg aerosol and 2 mg suspension aerosols, respectively. Bioavailability of suspension aerosols was fourfold greater than that of the solution aerosol. /Leuprolide acetate/ Metabolism / Metabolites Radiolabeling studies suggest that leuprolide is primarily metabolized to inactive penta-, tri-, and dipeptide entities, which are likely further metabolized. It is expected that various peptidases encountered throughout systemic circulation are responsible for leuprolide metabolism. Biological Half-Life Leuprolide has a terminal elimination half-life of approximately three hours. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Leuprolide has been associated with mild serum enzyme elevations during therapy in 3% to 5% of patients, but values above 3 times the upper limit of normal are rare, being reported in less than 1% of recipients. The serum enzyme elevations during leuprolide therapy have generally been transient and asymptomatic, resolving even with drug continuation and rarely requiring dose modification or discontinuation. Despite use for several decades, leuprolide has not been linked to convincing cases of clinically apparent liver injury. Routine monitoring of patients for liver test abnormalities is not recommended. Likelihood score: E (unlikely cause of clinically apparent liver injury). Protein Binding Leuprolide displays _in vitro_ binding to human plasma proteins between 43% and 49%. |
| 参考文献 | |
| 其他信息 |
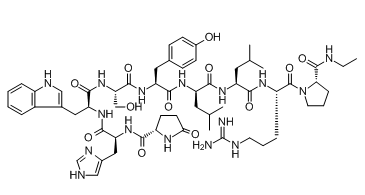
Leuprolide is an oligopeptide comprising pyroglutamyl, histidyl, tryptophyl, seryl, tyrosyl, D-leucyl, leucyl, arginyl, and N-ethylprolinamide residues joined in sequence. It is a synthetic nonapeptide analogue of gonadotropin-releasing hormone, and is used as a subcutaneous hydrogel implant (particularly as the acetate salt) for the treatment of prostate cancer and for the suppression of gonadal sex hormone production in children with central precocious puberty. It has a role as an antineoplastic agent, a gonadotropin releasing hormone agonist and an anti-estrogen.
Leuprolide is a synthetic 9-residue peptide analogue of gonadotropin-releasing hormone (GnRH). Unlike the endogenous decapeptide GnRH, leuprolide contains a single D-amino acid (D-leucyl) residue, which helps to increase its circulating half-life from three to four minutes to approximately three hours. As a GnRH mimic, leuprolide is capable of binding to the GnRH receptor (GnRHR) and inducing downstream modulation of both gonadotropin hormone and sex steroid levels. Prolonged activation of GnRHR results in significant downregulation of sex steroid levels, which is primarily responsible for the clinical efficacy of leuprolide in diverse conditions, including advanced prostate cancer, endometriosis, and central precocious puberty. Leuprolide was first approved in 1985 as a daily subcutaneous injection under the tradename Lupron™ by Abbvie Endocrine Inc. Since this initial approval, various long-acting intramuscular and subcutaneous products have been developed such that patients can be dosed once every six months. Leuprolide remains frontline therapy in all conditions for which it is indicated for use. Leuprolide is a Gonadotropin Releasing Hormone Receptor Agonist. The mechanism of action of leuprolide is as a Gonadotropin Releasing Hormone Receptor Agonist. Leuprolide is a parenterally administered, gonadotropin releasing hormone (GnRH) agonist which causes an inhibition of estrogen and androgen production and is used predominantly to treat advanced prostate cancer. Leuprolide has been associated with a modest rate of serum enzyme elevations during therapy, but has not been convincingly linked to instances of clinically apparent acute liver injury. Leuprolide is a synthetic nonapeptide analogue of gonadotropin-releasing hormone. Leuprolide binds to and activates gonadotropin-releasing hormone (GnRH) receptors. Continuous, prolonged administration of leuprolide in males results in pituitary GnRH receptor desensitization and inhibition of pituitary secretion of follicle stimulating hormone (FSH) and luteinizing hormone (LH), leading to a significant decline in testosterone production; in females, prolonged administration results in a decrease in estradiol production. This agent reduces testosterone production to castration levels and may inhibit androgen receptor-positive tumor progression. A potent synthetic long-acting agonist of GONADOTROPIN-RELEASING HORMONE that regulates the synthesis and release of pituitary gonadotropins, LUTEINIZING HORMONE and FOLLICLE STIMULATING HORMONE. See also: Leuprolide Acetate (has salt form); Leuprolide Mesylate (has salt form). Drug Indication Leuprolide is indicated for the treatment of advanced prostate cancer and as palliative treatment of advanced prostate cancer. It is also used for the treatment of pediatric patients with central precocious puberty (CPP). In combination with oral [norethisterone] (also known as norethindrone), leuprolide is also indicated for the initial treatment of the symptoms of endometriosis. Finally, in combination with iron supplementation, leuprolide is indicated for the preoperative hematological improvement of anemic patients with uterine leiomyomata (uterine fibroids). FDA Label Camcevi is indicated for the treatment of hormone dependent advanced prostate cancer and for the treatment of high-risk localised and locally advanced hormone dependent prostate cancer in combination with radiotherapy. Mechanism of Action Gonadotropin-releasing hormone (GnRH) is a naturally occurring decapeptide that modulates the hypothalamic-pituitary-gonadal (HPG) axis. GnRH binds to corresponding receptors (GnRHRs) on the anterior pituitary gonadotropes, which in turn release luteinizing hormone (LH) and follicle-stimulating hormone (FSH); these, in turn, affect the downstream synthesis and release of the sex hormones testosterone, dihydrotestosterone, estrone, and estradiol. Despite the variety of conditions indicated for treatment with leuprolide, the mechanism of action underlying efficacy is the same in all cases. As a GnRHR agonist, leuprolide binds to and initially activates downstream LH and FSH release; this initial spike in gonadotropin levels is responsible for some of the adverse effects associated with treatment. After 2-4 weeks of treatment, continuous stimulation of GnRHR results in feedback inhibition and significant downregulation of LH, FSH, and their corresponding downstream effects, producing a therapeutic benefit. These effects are reversible upon treatment discontinuation. Like naturally occurring luteinizing hormone-releasing hormone, initial or intermittent administration of leuprolide stimulates release of luteinizing hormone and follicle-stimulating hormone from the anterior pituitary. Luteinizing hormone and follicle-stimulating hormone release from the anterior pituitary transiently increases testosterone concentration in males. However, continuous administration of leuprolide in the treatment of prostatic carcinoma suppresses secretion of gonadotropin-releasing hormone, with a resultant fall in testosterone concentrations and a "medical castration". Initial stimulation of gonadotropins form the anterior pituitary is followed by prolonged suppression. Gonadotropin release from the anterior pituitary transiently increases estrone and estradiol concentrations in females. However, continuous administration of leuprolide in the treatment of endometriosis produces a fall in estrogens to postmenopausal levels. As a consequence of suppression of ovarian function, both normal and ectopic endometrial tissues become inactive and atrophic. As a result, amenorrhea occurs. |
| 分子式 |
C59H84N16O12
|
|---|---|
| 分子量 |
1209.42
|
| 精确质量 |
1208.645
|
| CAS号 |
53714-56-0
|
| 相关CAS号 |
74381-53-6 (monoacetate)
|
| PubChem CID |
657181
|
| 外观&性状 |
Fluffy solid
|
| 密度 |
1.4±0.1 g/cm3
|
| 熔点 |
150-155
|
| 折射率 |
1.682
|
| LogP |
0.41
|
| tPSA |
466.34
|
| 氢键供体(HBD)数目 |
15
|
| 氢键受体(HBA)数目 |
14
|
| 可旋转键数目(RBC) |
32
|
| 重原子数目 |
87
|
| 分子复杂度/Complexity |
2390
|
| 定义原子立体中心数目 |
9
|
| SMILES |
O=C([C@]([H])(C([H])([H])C([H])([H])C([H])([H])/N=C(\N([H])[H])/N([H])[H])N([H])C([C@]([H])(C([H])([H])C([H])(C([H])([H])[H])C([H])([H])[H])N([H])C([C@@]([H])(C([H])([H])C([H])(C([H])([H])[H])C([H])([H])[H])N([H])C([C@]([H])(C([H])([H])C1C([H])=C([H])C(=C([H])C=1[H])O[H])N([H])C([C@]([H])(C([H])([H])O[H])N([H])C([C@]([H])(C([H])([H])C1=C([H])N([H])C2=C([H])C([H])=C([H])C([H])=C12)N([H])C([C@]([H])(C([H])([H])C1=C([H])N=C([H])N1[H])N([H])C([C@]1([H])C([H])([H])C([H])([H])C(N1[H])=O)=O)=O)=O)=O)=O)=O)=O)N1C([H])([H])C([H])([H])C([H])([H])[C@@]1([H])C(N([H])C([H])([H])C([H])([H])[H])=O.O([H])C(C([H])([H])[H])=O
|
| InChi Key |
GFIJNRVAKGFPGQ-LIJARHBVSA-N
|
| InChi Code |
InChI=1S/C59H84N16O12/c1-6-63-57(86)48-14-10-22-75(48)58(87)41(13-9-21-64-59(60)61)68-51(80)42(23-32(2)3)69-52(81)43(24-33(4)5)70-53(82)44(25-34-15-17-37(77)18-16-34)71-56(85)47(30-76)74-54(83)45(26-35-28-65-39-12-8-7-11-38(35)39)72-55(84)46(27-36-29-62-31-66-36)73-50(79)40-19-20-49(78)67-40/h7-8,11-12,15-18,28-29,31-33,40-48,65,76-77H,6,9-10,13-14,19-27,30H2,1-5H3,(H,62,66)(H,63,86)(H,67,78)(H,68,80)(H,69,81)(H,70,82)(H,71,85)(H,72,84)(H,73,79)(H,74,83)(H4,60,61,64)/t40-,41-,42-,43+,44-,45-,46-,47-,48-/m0/s1
|
| 化学名 |
(2S)-N-[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2R)-1-[[(2S)-1-[[(2S)-5-(diaminomethylideneamino)-1-[(2S)-2-(ethylcarbamoyl)pyrrolidin-1-yl]-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]-5-oxopyrrolidine-2-carboxamide
|
| 别名 |
NSC-377526 NSC 377526 LeuprorelinA-43818 A 43818 A43818 NSC377526 leuprolide acetate Leuprorelinum Eligard
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.8268 mL | 4.1342 mL | 8.2684 mL | |
| 5 mM | 0.1654 mL | 0.8268 mL | 1.6537 mL | |
| 10 mM | 0.0827 mL | 0.4134 mL | 0.8268 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。