| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Oral absorption of lasmiditan is quick, with a median tmax of 1.8 hours. An open-label study looking at absorption pharmacokinetics found the Cmax and AUC0-t of lasmiditan following oral administration to be 322.8 ± 122.0 ng/mL and 1892 ± 746.0 ng.h/mL, respectively. The oral bioavailability of lasmiditan has been reported as approximately 40%. Co-administration of lasmiditan with a high-fat meal increased its Cmax and AUC by 22% and 19%, respectively, and delayed Tmax by approximately 1 hour - these differences in absorption are relatively minor and unlikely to be clinically significant. Similarly, severe renal impairment and mild-moderate hepatic impairment were found to increase both AUC and Cmax, but not to a clinically significant extent. Lasmiditan is eliminated primarily via metabolism, with renal excretion accounting for a small fraction of its total elimination. Of the small amount of drug found in the urine post-dose, approximately 66% is comprised of lasmiditan's S-M8 metabolite. Only 3% of an administered dose of lasmiditan was recovered unchanged in the urine, further implying a relatively extensive metabolism of this drug. Lasmiditan has been shown to penetrate the blood-brain barrier. Metabolism / Metabolites The hepatic and extra-hepatic metabolism of lasmiditan is catalyzed primarily by non-CYP enzymes, with ketone reduction appearing to be the primary pathway. While the specific enzymes involved in the metabolism of lasmiditan have not been elucidated, FDA labeling states that the following enzymes are _not_ involved in its metabolism: monoamine oxidases, CYP450 reductase, xanthine oxidase, alcohol dehydrogenase, aldehyde dehydrogenase, and aldo-keto reductases. The metabolites of lasmiditan have not been characterized in published research, but two of its metabolites (M7 and M18) are considered to be pharmacologically inactive. Biological Half-Life The mean elimination half-life of lasmiditan is 5.7 hours. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In preregistration controlled trials of lasmiditan in several thousand patients, mild-to-moderate serum aminotransferase elevations arose in a small percentage of patients (1% or less) and overall rates were not different from those in placebo recipients. In the controlled trials and subsequently with general use, there have been no reports of liver injury with symptoms or jaundice attributed to lasmiditan. Likelihood score: E (unlikely cause of clinically apparent acute liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation There is no published experience with lasmiditan during breastfeeding. If lasmiditan is required by the mother of an older infant, it is not a reason to discontinue breastfeeding, but until more data become available, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Lasmiditan exhibits a concentration-independent plasma protein binding of approximately 55-60%. |
| 参考文献 | |
| 其他信息 |
Pharmacodynamics
Lasmiditan belongs to a new and novel class of acute anti-migraine medications that exert their effects via inhibition of neuronal firing rather than vasoconstriction of cerebral arteries. Lasmiditan appears to have a relatively quick onset of action (an important characteristic in acute migraine treatment) with some patients reporting benefit within 20 minutes. Due to its ability to cause CNS depression (e.g. drowsiness, dizziness), lasmiditan may cause significant driving impairment and patients should be advised not to participate in activities requiring mental alertness for at least 8 hours after dosing. Lasmiditan may carry some potential for abuse and should be used with caution in patients who may be at risk of drug abuse - its controlled substance scheduling is currently under review in the United States by the Drug Enforcement Administration (DEA). The safety of lasmiditan in pregnancy is unknown and is currently being monitored with a pregnancy exposure registry created by Eli Lilly and Company. |
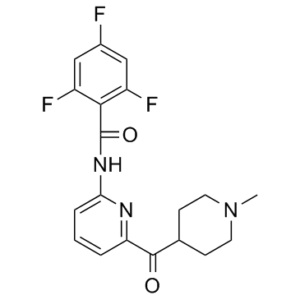
| 分子式 |
C19H18F3N3O2
|
|
|---|---|---|
| 分子量 |
377.38
|
|
| 精确质量 |
377.135
|
|
| CAS号 |
439239-90-4
|
|
| 相关CAS号 |
|
|
| PubChem CID |
11610526
|
|
| 外观&性状 |
Typically exists as solid at room temperature
|
|
| 密度 |
1.4±0.1 g/cm3
|
|
| 沸点 |
433.3±45.0 °C at 760 mmHg
|
|
| 闪点 |
215.9±28.7 °C
|
|
| 蒸汽压 |
0.0±1.0 mmHg at 25°C
|
|
| 折射率 |
1.585
|
|
| LogP |
1.9
|
|
| tPSA |
62.3
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
4
|
|
| 重原子数目 |
27
|
|
| 分子复杂度/Complexity |
530
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
FC1C([H])=C(C([H])=C(C=1C(N([H])C1=C([H])C([H])=C([H])C(C(C2([H])C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])C2([H])[H])=O)=N1)=O)F)F
|
|
| InChi Key |
XEDHVZKDSYZQBF-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C19H18F3N3O2/c1-25-7-5-11(6-8-25)18(26)15-3-2-4-16(23-15)24-19(27)17-13(21)9-12(20)10-14(17)22/h2-4,9-11H,5-8H2,1H3,(H,23,24,27)
|
|
| 化学名 |
2,4,6-trifluoro-N-[6-(1-methylpiperidine-4-carbonyl)pyridin-2-yl]benzamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6498 mL | 13.2492 mL | 26.4985 mL | |
| 5 mM | 0.5300 mL | 2.6498 mL | 5.2997 mL | |
| 10 mM | 0.2650 mL | 1.3249 mL | 2.6498 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04396574 | Recruiting | Drug: Lasmiditan | Migraine | Eli Lilly and Company | June 30, 2020 | Phase 3 |
| NCT04396236 | Recruiting | Drug: Lasmiditan Drug: Placebo |
Migraine | Eli Lilly and Company | June 15, 2020 | Phase 3 |
| NCT05903040 | Recruiting | Drug: Lasmiditan | Migraine Migraine With Aura Migraine Without Aura |
University of Florence | June 15, 2023 | N/A |
| NCT03988088 | Completed | Drug: Lasmiditan | Migraine | Eli Lilly and Company | July 22, 2019 | Phase 1 |
| NCT04881747 | Completed | Drug: Lasmiditan | Healthy | Eli Lilly and Company | May 14, 2021 | Phase 1 |
Proportion of migraine patients with headache relief (a decrease of headache from moderate or severe to none or mild) (HR) at 2h after intravenous lasmiditan (PBOplacebo).J Headache Pain.2012 Jun;13(4):271-5. |
|---|
Proportion of migraine patients with HR at 2h after oral lasmiditan 50–400mg (PBOplacebo).J Headache Pain.2012 Jun;13(4):271-5. |