| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
|
| 靶点 |
Endogenous Metabolite
|
|---|---|
| 体外研究 (In Vitro) |
在用二十碳五烯酸(EPA;100 μM;24 小时)处理的细胞中,C/EBPβ 的磷酸化形式明显可见,而在对照和 OA 或 LA 处理的 U937 细胞中几乎不明显 [1]。 H-Ras 和 N-Ras mRNA 水平在 1、3 和 24 小时后显着增加,并在 1 至 3 小时后持续增加。二十碳五烯酸对 K-Ras mRNA 水平没有影响 [1]。
先前的研究表明,饮食中的α-亚麻酸(ALA)会增加体内ω-3长链多不饱和脂肪酸(ω-3 LC-PUFAs)的水平,但其转化过程和相关基因仍知之甚少。在本研究中,我们设计了含有不同浓度ALA和二十碳五烯酸(EPA)的饮食来喂养小鼠。膳食ALA以线性方式增加了体内的ALA水平,也增加了ω-3 LC-PUFA浓度,但较高的ALA摄入量(超过5%)对体内ω-3 LC-FUFA水平没有额外影响。中等水平的膳食ALA增加了Fads1、Fads2和Elovl5等基因的表达,但较高水平的膳食丙氨酸(超过5%)抑制了它们在肝脏中的表达。进一步的研究表明,转化的EPA还可以以浓度依赖的方式抑制这些基因的表达,这表明Fads1、Fads2和Elovl5是参与ALA转化为ω-3 LC-PUFAs的关键基因。ALA内源性ω-3 LC-PUFA的生物合成受到底物水平、基因表达和产物抑制的影响[1]。 |
| 体内研究 (In Vivo) |
确定了241项研究,其中28项符合上述纳入标准,因此被纳入随后的荟萃分析。使用随机效应模型,与安慰剂相比,补充omega3-LC-PUFA后,总体标准化平均抑郁评分降低(标准化平均差异=-0.291,95%CI=-0.463至-0.120,z=-3.327,p=0.001)。然而,存在显著的异质性和发表偏倚的证据。荟萃回归研究显示,基线抑郁水平较高和补充DHAEPA比例较低对治疗效果有显著影响。亚组分析显示,对以下方面有显著影响:(1)诊断类别(双相情感障碍和重度抑郁症,与轻度至中度抑郁症、慢性疲劳和非临床人群相比,补充omega3-LC-PUFA后有显著改善);(2) 治疗性干预而非预防性干预;(3) 与单一疗法相反的辅助治疗;(4)补充类型。在使用纯DHA的3项研究中(标准化平均差0.001,95%置信区间-0.330至0.332,z=0.004,p=0.997),或在使用含有超过50%DHA的补充剂的4项研究中。相比之下,在13项使用含有超过50%EPA的补充剂的研究中(标准化平均差异=-0.446,95%CI=-0.753至-0.138,z=-2.843,p=0.005),以及在8项使用纯乙基EPA的研究中,抑郁症症状显著减轻(标准化平均差异=-0.396,95%CI=-0.650至-0.141,z=-3.051,p=0.002)。然而,进一步的元回归研究表明,疗效与研究方法质量、研究样本量和持续时间之间存在显著的负相关,从而限制了这些发现的置信度。结论:目前的荟萃分析提供了证据,表明EPA在治疗抑郁症方面可能比DHA更有效。然而,由于所纳入研究的局限性,需要更大、设计良好、持续时间足够长的随机对照试验来证实这些发现[1]
近视是全球视力受损和失明的主要原因。然而,目前还没有一种安全可行的近视控制和预防方法。在这里,我们研究了ω-3多不饱和脂肪酸(ω-3 PUFA)膳食补充剂对动物模型近视进展的治疗作用,以及对近距离工作引起的脉络膜血液灌注(ChBP)减少的治疗作用。近距离工作是年轻人近视的危险因素。我们证明,每天灌胃ω-3 PUFA(300mg二十二碳六烯酸[DHA]加60mg二十碳五烯酸[EPA])可显著减轻豚鼠和小鼠的形觉剥夺性近视的发展,以及豚鼠的晶状体诱导性近视。球周注射DHA也抑制了形态剥夺豚鼠的近视进展。豚鼠近视的抑制伴随着“ChBP减少巩膜缺氧级联反应”的抑制。此外,DHA或EPA治疗拮抗了培养的人巩膜成纤维细胞中缺氧诱导的肌成纤维细胞转分化。在人类受试者中,口服ω-3 PUFA部分缓解了近工作引起的ChBP下降。因此,这些动物和人类研究的证据表明,ω-3 PUFA是控制近视的潜在和现成的候选者[2]。 |
| 细胞实验 |
C/EBPβ和H-Ras-CpG岛的DNA分离和定量DNA甲基化分析[4]
使用FlexiGene DNA试剂盒提取对照U937细胞或用100µM OA或100µM二十碳五烯酸/EPA生长24小时的U937细胞的基因组DNA。EMBOSS和MethPrimer在线软件程序用于识别C/EBPβ、N-Ras和H-Ras基因的潜在CpG岛。使用Methyl Profiler qPCR引物分析定量人C/EBPβ(MePH25981-3A)和H-Ras(MePH14574-1A)的DNA甲基化水平。qRT-PCR程序按照手册说明进行。如前所述,使用限制性酶切(DNA甲基化酶试剂盒MeA-03)对CpG岛的DNA甲基化状态进行分析,然后进行基于SYBR Green的实时PCR检测。使用ΔCt法计算每个DNA组分(甲基化和非甲基化)的相对量。 亚硫酸氢盐修饰基因组DNA和测序[4] 使用FlexiGene DNA试剂盒从U937细胞、对照细胞或用100µM OA或100µM二十碳五烯酸/EPA培养24小时的细胞中获得基因组DNA。如前所述,进行亚硫酸氢盐反应以确定DNA甲基化状态。使用以下引物通过PCR扩增覆盖N-Ras-CpG岛(-29/+171)和H-Ras-CpG-岛B(640/882)的DNA片段。N-Ras:为5′-AAAGTTTTTGTGTGTGTGAGATTAGTAA-3′;修订版,5′-TTAAACAATTTAAACCACACC-3′。H-Ras:为5′-AGTTTTTTTGGTTGAAAGATGT-3′;rev,5′-ACACCCAAATTAACATAATC-3′。将PCR产物克隆到pCR2.1 TOPO中,并在Genechron Ylichron实验室使用T7引物对从两个独立PCR中随机挑选的六个克隆进行测序。 染色质免疫沉淀[4] 使用EZ-ChIP试剂盒对U937细胞(约106个)进行ChIP检测,对照或用100µM OA或二十碳五烯酸/EPA培养24小时。细胞被交联,细胞裂解物被超声处理,直到染色质片段的大小变为200-1.000 bp。使用小鼠RNAPII 8WG16单克隆抗体MMS-126R或兔p53抗体#928进行免疫沉淀。小鼠或兔IgG用作阴性对照。免疫沉淀后,用Brilliant SYBR Green qPCR Master Mix对回收的染色质样本进行qRT-PCR。在RNAPII检测中,扩增的H-Ras序列位于i)外显子1(1/+135),ii)内含子1区域C(+136/+639),iii)CpG岛B(+640/+882),iv)内含子一区域D(+883/+1167)和v)外显体2(+1168/+1331)内。使用了以下引物。i) 外显子1:为5′-TGCCCCTGCCCGCAACCGAG-3′;修订版,5′-CGTTCACAGGGCGACTGCC-3′。ii)内含子1区C:为5′-GTGAACGGGTGAGGGCA-3′;修订版,5′-CGCGCGCGCGTATTGCTGC-3′。iii)CpG岛B:为5′-CCGTTTCTGGAGAGCGGTAA-3′;修订版,5′-GTCGGAGAAGGCTAAAGG-3′。iv)内含子1区D:5′-TCAGATGGCCCTGCCAGAG-3′;rev,5′-TTCCTACAGGGTCTCCTG-3′。v) 外显子2:for,5′CAGGAGACCTGTAGAGGA-3′;5′-GGATCAGCTGGATGGTCAGC-3′。在p53检测中,使用以下引物扩增含有CpG岛B p53元件的序列:for,5′-CGCTCAAAAATACTTGTCGG-3′;版次:5′-TTACCGTCCAGACAGG-3′。根据公式2-Δ[C(IP)-C(输入)]-2-Δ[C(对照IgG)-C(输出)]对数据进行定量分析。 |
| 动物实验 |
Six-week-old male C57BL/6J mice were used. After one week of acclimatization with free access to standard mouse chow (commercial diet, 17.14% of energy from fat, 5.05 g/100 g) and water, the mice were randomly divided into nine groups each containing six mice and fed ALA series diets (1, 2.5, 5, or 7.5 wt%), 5% ALA and EPA series diets (0.25, 0.5, 1 wt%), EPA diet (2 wt%), or the control diet (Ctl diet: depleted in ω-3 PUFA) for seven weeks. The diet ingredients were shown in ESI Tables S1 and S2.† All animals were maintained in barrier cages and fed with the appropriate special diet restricted to 10 g per mouse per day.[3]
|
| 药代性质 (ADME/PK) |
Metabolism / Metabolites
Eicosapentaenoic acid has known human metabolites that include Juniperonic acid. |
| 参考文献 |
|
| 其他信息 |
All-cis-5,8,11,14,17-icosapentaenoic acid is an icosapentaenoic acid having five cis-double bonds at positions 5, 8, 11, 14 and 17. It has a role as a nutraceutical, a micronutrient, an antineoplastic agent, an antidepressant, a Daphnia galeata metabolite, a mouse metabolite, an anticholesteremic drug and a fungal metabolite. It is an icosapentaenoic acid and an omega-3 fatty acid. It is a conjugate acid of an all-cis-5,8,11,14,17-icosapentaenoate.
Important polyunsaturated fatty acid found in fish oils. It serves as the precursor for the prostaglandin-3 and thromboxane-3 families. A diet rich in eicosapentaenoic acid lowers serum lipid concentration, reduces incidence of cardiovascular disorders, prevents platelet aggregation, and inhibits arachidonic acid conversion into the thromboxane-2 and prostaglandin-2 families. Eicosapentaenoic acid has been reported in Mortierella alpina, Tornabea scutellifera, and other organisms with data available. Icosapent is the free fatty acid (FFA) form of eicosapentaenoic acid (EPA), a polyunsaturated long-chain fatty acid found in fish oil with a 20-carbon backbone and 5 double bonds, with potential supplementing, anti-inflammatory, anti-thrombotic, immunomodulating, anti-angiogenic and chemopreventive activities. Upon administration of icosapent, the free form of EPA is incorporated in cell membrane phospholipids and replaces arachidonic acid. This inhibits arachidonic acid conversion into thromboxanes and prostaglandin E2 (PGE2). Upon oral administration of icosapent, the EPA-FFA prevents and suppresses colonic neoplasia and reduces polyp formation and growth through as of yet not fully elucidated mechanisms. Important polyunsaturated fatty acid found in fish oils. It serves as the precursor for the prostaglandin-3 and thromboxane-3 families. A diet rich in eicosapentaenoic acid lowers serum lipid concentration, reduces incidence of cardiovascular disorders, prevents platelet aggregation, and inhibits arachidonic acid conversion into the thromboxane-2 and prostaglandin-2 families. See also: Icosapent Ethyl (active moiety of); Fish Oil (is active moiety of); Eicosapentaenoic Acid (subclass of) ... View More ... Drug Indication EPA can be used for lowering elevated triglycerides in those who are hyperglyceridemic. In addition, EPA may play a therapeutic role in patients with cystic fibrosis by reducing disease severity and may play a similar role in type 2 diabetics in slowing the progression of diabetic nephropathy. FDA Label Treatment of Familial Adenomatous Polyposis Mechanism of Action The anti-inflammatory, antithrombotic and immunomodulatory actions of EPA is probably due to its role in eicosanoid physiology and biochemistry. Most eicosanoids are produced by the metabolism of omega-3 fatty acids, specifically, arachidonic acid. These eicosanoids, leukotriene B4 (LTB4) and thromboxane A2 (TXA2) stimulate leukocyte chemotaxis, platelet aggregation and vasoconstriction. They are thrombogenic and artherogenic. On the other hand, EPA is metabolized to leukotriene B5 (LTB5) and thromboxane A3 (TXA3), which are eicosanoids that promote vasodilation, inhibit platelet aggregation and leukocyte chemotaxis and are anti-artherogenic and anti-thrombotic. The triglyceride-lowering effect of EPA results from inhibition of lipogenesis and stimulation of fatty acid oxidation. Fatty acid oxidation of EPA occurs mainly in the mitochondria. EPA is a substrate for Prostaglandin-endoperoxide synthase 1 and 2. It also appears to affect the function and bind to the Carbohydrate responsive element binding protein (ChREBP) and to a fatty acid receptor (G-coupled receptor) known as GP40. Pharmacodynamics Eicosanoids are chemical messengers derived from 20-carbon polyunsaturated fatty acids that play critical roles in immune and inflammatory responses. Both 20-carbon omega-6 fatty acids (arachidonic acid) and 20-carbon omega-3 fatty acids (EPA) can be found in cell membranes. During an inflammatory response, arachidonic acid and EPA are metabolized by enzymes known as cyclooxygenases and lipoxygenases to form eicosanoids. Increasing omega-3 fatty acid intake increases the EPA content of cell membranes and decreases the arachidonic acid content, resulting in higher proportions of eicosanoids derived from EPA. Physiologic responses to arachidonic acid-derived eicosanoids differ from responses to EPA-derived eicosanoids. In general, eicosanoids derived from EPA are less potent inducers of inflammation, blood vessel constriction, and clotting than eicosanoids derived from arachidonic acid. |
| 分子式 |
C20H30O2
|
|---|---|
| 分子量 |
302.451
|
| 精确质量 |
302.224
|
| CAS号 |
10417-94-4
|
| 相关CAS号 |
Eicosapentaenoic Acid-d5;1197205-73-4;Eicosapentaenoic acid ethyl ester;86227-47-6;Eicosapentaenoic Acid sodium;73167-03-0
|
| PubChem CID |
446284
|
| 外观&性状 |
Colorless to light yellow liquid
|
| 密度 |
0.9±0.1 g/cm3
|
| 沸点 |
439.3±24.0 °C at 760 mmHg
|
| 熔点 |
-54--53ºC(lit.)
|
| 闪点 |
336.0±18.0 °C
|
| 蒸汽压 |
0.0±2.3 mmHg at 25°C
|
| 折射率 |
1.513
|
| LogP |
6.23
|
| tPSA |
37.3
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
13
|
| 重原子数目 |
22
|
| 分子复杂度/Complexity |
398
|
| 定义原子立体中心数目 |
0
|
| SMILES |
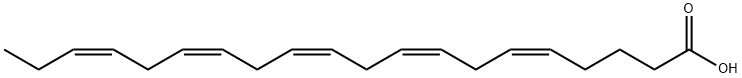
CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(O)=O
|
| InChi Key |
JAZBEHYOTPTENJ-JLNKQSITSA-N
|
| InChi Code |
InChI=1S/C20H30O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22/h3-4,6-7,9-10,12-13,15-16H,2,5,8,11,14,17-19H2,1H3,(H,21,22)/b4-3-,7-6-,10-9-,13-12-,16-15-
|
| 化学名 |
(5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaenoic acid
|
| 别名 |
EPA. Icosapent, Eicosapentaenoic acid; Timnodonic acid; Icosapent; 10417-94-4; Icosapentaenoic acid; EPA; cis-5,8,11,14,17-Eicosapentaenoic acid; Icosapento; Eicosapentaenoic acid, Timnodonic acid, Vascepa, Epadel, EPAX
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
Ethanol :≥ 100 mg/mL (~330.63 mM)
DMSO : ≥ 30 mg/mL (~99.19 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.27 mM) (饱和度未知) in 10% EtOH + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清乙醇储备液添加到 900 μL 玉米油中并混合均匀。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.08 mg/mL (6.88 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: 2.08 mg/mL (6.88 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 配方 4 中的溶解度: ≥ 2.08 mg/mL (6.88 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,您可以将 100 μL 20.8 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.3063 mL | 16.5317 mL | 33.0633 mL | |
| 5 mM | 0.6613 mL | 3.3063 mL | 6.6127 mL | |
| 10 mM | 0.3306 mL | 1.6532 mL | 3.3063 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。