| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 50g |
|
||
| 100g |
|
||
| 200g |
|
||
| Other Sizes |
|

| 靶点 |
Human Endogenous Metabolite; Microbial Metabolite
|
|---|---|
| 体外研究 (In Vitro) |
叶酸处理以剂量依赖性方式增加 HepG2、Huh-7D12、Hs578T 和 JURKAT 细胞中 BRCA1 mRNA 的表达,以及 HepG2、Hs578T、MCF7 和 MDA-MB-157 细胞中 BRCA2 mRNA 的表达。 FA 对任何卵巢细胞系或相应的正常细胞没有影响。在Hs578T细胞中,叶酸增加BRCA1蛋白表达,但不增加HepG2细胞表达;未检测到 BRCA2 蛋白表达。虽然 FA 治疗对乳腺细胞有短期影响,但对肝细胞 DNA 修复没有影响。 FA 处理对 BRCA1 或 BRCA2 DNA 的甲基化没有影响,但某些细胞系在特定 CpG 位点具有不同水平的甲基化[1]。
|
| 体内研究 (In Vivo) |
无叶酸(1、5 mg/kg;界面)会阻止适应新环境的小鼠染色体后代基因表达的表观遗传修饰[3]。叶酸(10、50、100 mg/kg;侧)在此行为小鼠模型中表现出抗抑郁样作用 [2]。
|
| 细胞实验 |
根据制造商的说明,在 TRI Reagent 中收获之前,所有细胞系均用 0、25、50、75 或 100 nmol/L FA 处理 72 小时,以确定 FA 补充对 BRCA1 和 BRCA2 mRNA 表达的影响。
|
| 动物实验 |
Animal/Disease Models: 30-40 g Swiss mice [2]
Doses: 10, 50, 100 mg/kg Route of Administration: Oral Experimental Results: diminished immobility time in the forced swim test (FST) (F324=11.21), and Immobility time in the tail suspension test (TST) had a significant effect (F3, 20=5.71). Animal/Disease Models: 30-40 g Swiss mice [2] Doses: 1-10 nmol/site Route of Administration: Intracerebroventricular injection Experimental Results: diminished mouse FST (F3,22=12.31) and TST (F3,22=5.50) immobile time). Animal/Disease Models: Virgin female Wistar rats [3] Doses: 1, 5 mg/kg (180 g/kg protein plus 1 mg/kg folic acid or 90 g/kg casein plus 1, 5 mg/kg folic acid) Route of Administration: Oral administration Experimental Results: Prevention of epigenetic modifications in liver gene expression in offspring. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Folic acid is absorbed rapidly from the small intestine, primarily from the proximal portion. Naturally occurring conjugated folates are reduced enzymatically to folic acid in the gastrointestinal tract prior to absorption. Folic acid appears in the plasma approximately 15 to 30 minutes after an oral dose; peak levels are generally reached within 1 hour. After a single oral dose of 100 mcg of folic acid in a limited number of normal adults, only a trace amount of the drug appeared in the urine. An oral dose of 5 mg in 1 study and a dose of 40 mcg/kg of body weight in another study resulted in approximately 50% of the dose appearing in the urine. After a single oral dose of 15 mg, up to 90% of the dose was recovered in the urine. A majority of the metabolic products appeared in the urine after 6 hours; excretion was generally complete within 24 hours. Small amounts of orally administered folic acid have also been recovered in the feces. Folic acid is also excreted in the milk of lactating mothers. Tetrahydrofolic acid derivatives are distributed to all body tissues but are stored primarily in the liver. Folic acid is absorbed rapidly from the GI tract following oral administration oral administration; the vitamin is absorbed mainly in the proximal portion of the small intestine. The monoglutamate forms of folate, including folic acid, are transported across the proximal small intestine via a saturable pH-dependent process. Higher doses of the pteroylmonoglutamates, including folic acid, are absorbed via a nonsaturable passive diffusion process. The efficiency of absorption of the pteroylmonoglutamates is greater than that of pteroylpolyglutamates. Following oral administration, peak folate activity in blood occurs within 30 to 60 minutes. Synthetic folate is almost 100% bioavailable when administered in fasting individuals. While the bioavailability of naturally occurring folate in food is about 50%, bioavailability of synthetic folic acid consumed with a meal ranges from 85 to 100%. Approximately two-thirds of folate in plasma is protein bound. ... When pharmacologic doses of folic acid are administered, a significant amount of unchanged folic acid is found in the plasma. The liver contains more than 50% of the body stores of folate, or about 6 to 14 milligrams. The total body store of folate is about 12 to 28 miligrams. For more Absorption, Distribution and Excretion (Complete) data for FOLIC ACID (11 total), please visit the HSDB record page. Metabolism / Metabolites Folic acid is metabolized in the liver into the cofactors dihydrofolate (DHF) and tetrahydrofolate (THF) by the enzyme dihydrofolate reductase (DHFR). Folic acid is converted (in the presence of ascorbic acid) in the liver and plasma to its metabolically active form (tetrahydrofolic acid) by dihydrofolate reductase. Following absorption of 1 mg or less, folic acid is largely reduced and methylated in the liver to N-methyltetrahydrofolic acid... . The folates are taken up by the liver and metabolized to polyglutamate derivatives (principally pteroylpentaglutamate), via the action of folypolyglutamate synthase. ... Folate polyglutamates are released from the liver to the systemic circulation and to the bile. When released from the liver into the circulation, the polyglutamate forms are hydrolyzed by gamma-glutamylhydrolase and reconverted to the monoglutamate forms. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION: Folic acid is an antianaemic vitamin. Origin of the substance: Folic acid was isolated from green leafy vegetables, liver, yeast and fruits. Synthetic folic acid is commercially available. Yellow to orange brown crystalline powder which is odorless. Readily soluble in alkali, hydroxides and carbonates. Insoluble in alcohol, acetone, chloroform and ether. Solutions are inactivated by ultraviolet light. Alkaline solutions are sensitive to oxidation and acid solutions are sensitive to heat. Indications: For the prevention and treatment of vitamin B deficiency. For the treatment of megaloblastic anemia and macrocytic anemia due to folic acid deficiency. Folic acid supplements may be required in low birth weight infants, infants breastfed by folic acid deficient mothers, or those with prolonged diarrhea and infection. Other conditions which may increase folic acid requirements include alcoholism, hepatic disease, hemolytic anemia, lactation, oral contraceptive use and pregnancy. It has been given to pregnant mothers to reduce the risk of birth defects. HUMAN EXPOSURE: Main risks and target organs: Folic acid is relatively non-toxic. However, there have been reports of reactions to parenteral injections. Allergic reactions to folic acid have been rarely reported. Summary of clinical effects: Severe allergic reactions are characterized by hypotension, shock, bronchospasm, nausea, vomiting, rash, erythema. Itching may also occur. Adverse gastrointestinal and central nervous system effects have been reported. Treatment with folic acid is usually well tolerated except for rare reports of allergic reactions. Bioavailability: Folic acid is rapidly absorbed from gastrointestinal tract following oral administration. Peak folate activity in blood is 30 to 60 minutes after oral administration. Contraindications: It should be given with caution to patients with abnormal renal function. It is also contra-indicated in patients who show hypersensitivity reactions to folic acid. Caution is advised in patients who may have folate dependent tumours. Folic acid should never be given alone or in conjunction with inadequate amounts of Vitamin B12 for the treatment of undiagnosed megaloblastic anaemia. Although folic acid may produce a haematopoietic response in patients with megaloblastic anaemia due to Vitamin B12, it fails to prevent the onset of subacute combined degeneration of the cord. Absorption by route of exposure: Oral: Folic acid is rapidly absorbed from the proximal part of the gastrointestinal tract following oral administration. It is mainly absorbed in the proximal portion of the small intestine. The naturally occurring folate polyglutamate is enzymatically hydrolyzed to monoglutamate forms in the gastrointestinal tract prior to absorption. The peak folate activity in blood after oral administration is within 30 to 60 minutes. Enterohepatic circulation of folate has been demonstrated. Distribution by route of exposure: Tetrahydrofolic acid and its derivatives are distributed in all body tissues. The liver contains half of the total body stores of folate and is the principal storage site. Metabolism: Folic acid once absorbed is acted upon by hepatic dihydrofolate reductase to convert to its metabolically active form which is tetrahydrofolic acid. Following absorption, folic acid is largely reduced and methylated in the liver to N-5 methyltetrahydrofolic acid, which is the main transporting and storage form of folate in the body. Larger doses may escape metabolism by the liver and appear in the blood mainly as folic acid. Elimination by route of exposure: Oral: Following oral administration of single doses of folic acid in health adults, only a trace amount of the drug appears in urine. Following administration of large doses, the renal tubular reabsorption maximum is exceeded and excess folate is excreted unchanged in urine. Small amounts of orally administered folic acid have been recovered from feces. Pharmacodynamics: Folic acid is transformed into different coenzymes that are responsible for various reactions of intracellular metabolism mainly conversion of homocysteine to methionine, conversion of serine to glycine, synthesis of thymidylate, histidine metabolism, synthesis of purines and utilization or generation of formate. In man, nucleoprotein synthesis and the maintenance of normal erythropoiesis requires exogenous folate. Folic acid is the precursor of tetrahydrofolic acid which is active and acts as a co-factor for 1-carbon transfer reactions in the biosynthesis of purines and thymidylates of nucleic acids. Adults: There is little data available on folic acid toxicity in humans. A case of two patients who showed exacerbation of psychotic behavior during treatment with folic acid has been reported. Cytomorphological effects of folic acid were studied using in-vitro establishment human oral epithelium. A concentration twice that used clinically did not induce marked cytotoxic reaction in cultured cells. The most pronounced changes were cultures which showed degenerating cells showing edema, increased translucency of the cytoplasm, flattened cells and atypical filaments. Interactions: Folic acid therapy may increase phenytoin metabolism in folate deficient patients resulting in decreased phenytoin serum concentration. It has also been reported that concurrent administration of folic acid and chloramphenicol in folate deficient patients may result in antagonism of the hematopoietic response to folic acid. The use of ethotoin or mephenytoin concurrently with folic acid may decrease the effects of hydantoins by increasing hydantoin metabolism. Trimethoprim acts as a folate antagonist by inhibiting dihydrofolate reductase, so in patients receiving this drug leucovorin calcium must be given instead of folic acid. Folic acid may also interfere with the effects of pyrimethamine. Aminopterin (4 aminofolic acid) and methotrexate (4 amino- 10 methylfolic acid) antagonizes reduction of folic acid to tetrahydrofolic acid. Methotrexate continues to be used as an antineoplastic drug whose activity may be dependent on blocking certain syntheses, of purines, in which folic acid is required, thereby depriving neoplastic cells of compounds essential for their proliferation. Calcium leucovorin is used therapeutically as a potent antidote for the toxic effects of folic acid antagonists used as antineoplastic agents. Methotrexate or pyrimethamine or triamterene also acts as folate antagonist by inhibiting dihydrofolic reductase. Analgesics, anticonvulsants, antimalarials and corticosteroids may cause folic acid deficiency. Main adverse effects: Allergic reactions to folic acid have been rarely reported including erythema, rash, itching, general malaise and bronchospasm. Adverse gastrointestinal and central nervous system effects have been reported in patients receiving 15 mg of folic acid daily for one month. ANIMAL/PLANT STUDIES: Mode of action: Folic acid is relatively non-toxic. Toxicity studies in mice showed that folic acid could cause convulsions, ataxia and weakness. Histopathological studies in some strains of mice showed that toxic doses may also cause acute renal tubular necrosis. A possible relationship between folic acid neurotoxicity and cholinergic receptors in the pyriform cortex and amygdala has been shown. Interactions The use of high dose folic acid concomitantly with pyrimethamine to prevent bone marrow depression may cause a pharmacodynamic antagonism of the antiparasitic effect of pyrimethamine. Nonsteroidal antiinflammatory drugs (NSAIDS), including ibuprofen, indomethacin, naproxen, mefenamic acid, piroxicam, and sulindac taken at high therapeutic dosages may exert antifolate activity. Folic acid supplementation in mice was found to augment the therapeutic activity and ameliorate the adverse reactions of the ... antifolate cancer chemotherapeutic agent lometrexol. The /daily/ use of folic acid ... was found to enhance the antidepressant action of fluoxetine ... For more Interactions (Complete) data for FOLIC ACID (17 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Hematinics Folic acid is indicated for prevention and treatment of folic acid deficiency states , including megaloblastic anemia and anemias of nutritional origin, pregnancy, infancy, or childhood. Recommended intakes may be increased and /or supplementation may be necessary in the following persons or conditions (based on documented folic acid deficiency): Alcoholism, hemolytic anemia, chronic fever, gastrectomy, chronic hemodialysis, infants (low birth weight, breast-fed, or those receiving unfortified formulas such as evaporated milk or goats milk), Intestinal disease (celiac disease, tropical sprue, persistent diarrhea), malabsorption syndromes associated with hepatic-biliary disease (hepatic function impairment, alcoholism with cirrhosis), /and/ prolonged stress. MEDICATION (VET): ... To prevent macrocytic anemia, embryonic death, cervical paralysis, and perosis In chicks. For more Therapeutic Uses (Complete) data for FOLIC ACID (7 total), please visit the HSDB record page. Drug Warnings Allergic reactions to folic acid preparations have been reported rarely and have included erythema, rash, itching, general malaise, and bronchospastic respiratory difficulty. Adverse GI effects such as anorexia, nausea, abdominal distention, flatulence, and a bitter/bad taste and adverse CNS effects such as altered sleep patterns, difficulties concentrating, irritability, overactivity, excitement, mental depression, confusion, and impaired judgement have been reported rarely in patients receiving 15 mg of folic acid daily for one month. Decreased serum vitamin B12 concentration may occur in patients receiving prolonged folic acid therapy. Folic acid should be administered with extreme caution in patients with undiagnosed anemia, since folic acid may obscure the diagnosis of pernicious anemia by alleviating hematologic manifestations of the disease while allowing the neurologic complications to progress. This may result in severe nervous system damage before the correct diagnosis is made. For more Drug Warnings (Complete) data for FOLIC ACID (7 total), please visit the HSDB record page. Pharmacodynamics Folic acid is a water-soluble B-complex vitamin found in foods such as liver, kidney, yeast, and leafy, green vegetables. Also known as folate or Vitamin B9, folic acid is an essential cofactor for enzymes involved in DNA and RNA synthesis. More specifically, folic acid is required by the body for the synthesis of purines, pyrimidines, and methionine before incorporation into DNA or protein. Folic acid is the precursor of tetrahydrofolic acid, which is involved as a cofactor for transformylation reactions in the biosynthesis of purines and thymidylates of nucleic acids. Impairment of thymidylate synthesis in patients with folic acid deficiency is thought to account for the defective deoxyribonucleic acid (DNA) synthesis that leads to megaloblast formation and megaloblastic and macrocytic anemias. Folic acid is particularly important during phases of rapid cell division, such as infancy, pregnancy, and erythropoiesis, and plays a protective factor in the development of cancer. As humans are unable to synthesize folic acid endogenously, diet and supplementation is necessary to prevent deficiencies. In order to function properly within the body, folic acid must first be reduced by the enzyme dihydrofolate reductase (DHFR) into the cofactors dihydrofolate (DHF) and tetrahydrofolate (THF). This important pathway, which is required for de novo synthesis of nucleic acids and amino acids, is disrupted by anti-metabolite therapies such as [DB00563] as they function as DHFR inhibitors to prevent DNA synthesis in rapidly dividing cells, and therefore prevent the formation of DHF and THF. In general, folate serum levels below 5 ng/mL indicate folate deficiency, and levels below 2 ng/mL usually result in megaloblastic anemia. |
| 分子式 |
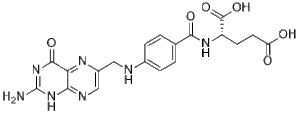
C19H19N7O6
|
|---|---|
| 分子量 |
441.3975
|
| 精确质量 |
441.14
|
| 元素分析 |
C, 51.70; H, 4.34; N, 22.21; O, 21.75
|
| CAS号 |
59-30-3
|
| 相关CAS号 |
70114-87-3 (Folic acid, methyl-); 134-05-4 (10-Formylfolic acid); 54931-98-5 (10-Thiofolic acid); 119770-54-6 (11-Deazahomofolic acid); 72254-43-4 (11-Oxahomofolic acid); 85597-18-8 ( 5,10-Dideazafolic acid); 111113-75-8 (5,6,7,8-Tetrahydro-8-deazahomofolic acid); 130327-67-2 ( 5-Deazaisofolic acid); 51989-25-4 ( 8-Deazafolic acid; NSC 173522); 111113-73-6 (8-Deazahomofolic acid); 14866-11-6 (Dihydrohomofolic acid); 83704-88-5 (HH-Folic acid is a derivative of vitamin B); 3566-25-4 (Homofolic acid); 11076-68-9 (Lyofolic acid); 29291-35-8 (Nitrosofolic acid; CCRIS 466); 88912-57-6 (Pyrrofolic acid); 135-16-0 (5,6,7,8-tetrahydrofolic acid; Tetrahydropteroylglutamic acid; th-folate; folate-H4); 5786-82-3 (Tetrahydrohomofolic acid); 52454-37-2 (10-Deazaaminopterin; 10-Deaza-aminopterin; NSC 311469; NSC-311469; NSC311469); 28459-40-7 (10-Formyldihydrofolate); 2800-34-2 (10-Formyltetrahydrofolic acid; 10-Formyl-THF; 10FTHF); 74163-10-3 ( 11-Thiohomoaminopterin, a close analog of 11-thiohomofolic acid); 85803-29-8 (2-Fluoroaminopterin); 5472-96-8 (3-Chloromethotrexate); 59904-24-4 (5-Methyldihydrofolate); 50998-20-4 (5-Methyltetrahydrofolate triglutamate); 73951-54-9 (6R-Leucovorin); 77739-71-0 (Acanthifolicin); 25312-31-6 (Aminoanfol, an antifolic acid compound); 31690-11-6 (Arfolitixorin free, an antifolate modulator); 149930-93-8 (Arfolitixorin sulfate); 154705-24-5 (Arfolitixorin sodium); 501332-69-0 (BGC-945); 115940-48-2 (Calcium dextrofolinate, calcium salt of a derivative of Folic Acid);26560-38-3 (Calcium methyltetrahydrofolate); 5854-11-5 (CB 3705; CB-3705; CB3705; 5,8-Dideazafolic acid); 76849-19-9 (CB 3717; CB-3717; CB3717; N(sup 10)-Propargyl-5,8-dideazafolic acid); 18921-73-8 (chlorasquin, an inhibitor of dihydrofolate reductase); 4033-27-6 (Dihydrofolate); 36093-88-6 (Dihydroaminopterin); 528-74-5 (Dichloromethotrexate); 6807-82-5 ( Diopterin, a folic acid analog); 1148151-21-6 [Folitixorin calcium, (6R)-]; 6484-89-5 (Folate sodium; Folvite sodium); 815587-59-8 [olitixorin calcium, (6S)-]; 133978-76-4 (Folitixorin sodium); 35409-55-3 (Hexaglutamate folate); 112887-62-4 (ICI 198583, an antifolate thymidylate synthase inhibitor); 31690-09-2 (Levomefolinic acid); 1423663-76-6 ( Levomefolate sodium); 1429498-11-2 ( Levomefolate magnesium); 58-05-9 ( Levoleucovorin free acid); 1492-18-8 ( Levoleucovorin calcium); 6035-45-6 ( Levoleucovorin calcium hydrate); 163254-40-8 ( Levoleucovorin sodium); 1141892-29-6 (Levoleucovorin sodium); 120408-07-3 (Lometrexol sodium); 106400-81-1 (Lometrexol free acid); 106400-18-4 (LY249543, the S-isomer of lometrexol); 136208-85-0 (LY249543, the S-isomer of lometrexol); 82339-36-4 (Lysine-iodoacetylmethotrexate, a Folic Acid Antagonist); 7413-34-5 ( Methotrexate disodium); 7532-09-4 (Methotrexate monosodium); 59-05-2 (Methotrexate free acid); 6745-93-3 (Methotrexate hydrate); 15475-56-6 (Methotrexate sodium); 66147-29-3 (Methotrexate 1-methyl ester); 67022-39-3 (Methotrexate 5-methyl este); 79573-48-1 (Mefox; (6RS)-Mefox); 2179-16-0 (Ninopterin); 41600-13-9 (NSC269401, the isotope labelled analog of Methotrexate Diglutamate; 41600-14-0 ( NSC341076 is the isotope labelled analog of Methotrexate Triglutamate); 2197232-28-1 (OSI-7904L,1843U89; racemic) 139987-54-5 (OSI-7904L,1843U89; free acid); 89-38-3 (Pteropterin); 6164-84-7 (Pteropterin monohydrate); 33611-85-7 ( Pteroylpentaglutamic acid); 112887-68-0 (Raltitrexed); 4299-28-9 (Tetrahydromethotrexate); 29701-38-0 (Triglutamate folate); 52128-35-5 (Trimetrexate)
|
| PubChem CID |
135398658
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| 熔点 |
482 °F (decomposes) (NTP, 1992)
250 °C |
| LogP |
-1.1
|
| tPSA |
209
|
| 氢键供体(HBD)数目 |
6
|
| 氢键受体(HBA)数目 |
10
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
32
|
| 分子复杂度/Complexity |
767
|
| 定义原子立体中心数目 |
1
|
| SMILES |
C1=CC(=CC=C1C(=O)N[C@@H](CCC(=O)O)C(=O)O)NCC2=CN=C3C(=N2)C(=O)NC(=N3)N
|
| InChi Key |
OVBPIULPVIDEAO-LBPRGKRZSA-N
|
| InChi Code |
InChI=1S/C19H19N7O6/c20-19-25-15-14(17(30)26-19)23-11(8-22-15)7-21-10-3-1-9(2-4-10)16(29)24-12(18(31)32)5-6-13(27)28/h1-4,8,12,21H,5-7H2,(H,24,29)(H,27,28)(H,31,32)(H3,20,22,25,26,30)/t12-/m0/s1
|
| 化学名 |
(2S)-2-[[4-[(2-amino-4-oxo-3H-pteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid
|
| 别名 |
FA; N-(4-{[(2-amino-4-oxo-1,4-dihydropteridin-6-yl)methyl]amino}benzoyl)-L-glutamic acid; pteroyl-L-glutamic acid; folacin; pteroyl-L-glutamate; Folic acid; Vitamin B11; Vitamin B9; Vitamin Bc; Vitamin Be; Vitamin M
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
1M NaOH: ~100 mg/mL (~226.6 mM)
DMSO: ~33.3 mg/mL (~75.5 mM) H2O: < 0.1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.71 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.08 mg/mL (4.71 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2655 mL | 11.3276 mL | 22.6552 mL | |
| 5 mM | 0.4531 mL | 2.2655 mL | 4.5310 mL | |
| 10 mM | 0.2266 mL | 1.1328 mL | 2.2655 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03853304 | Active Recruiting |
Other: + Folic acid Other: + Vitamin B12 |
Anemia Folate Deficiency |
Cornell University | October 1, 2023 | Not Applicable |
| NCT05959044 | Recruiting | Drug: Folic Acid Tablet Other: Placebo |
Parkinson Disease | Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh |
April 16, 2023 | Phase 2 |
| NCT05296369 | Recruiting | Drug: Folic acid | Lung Cancer | Guizhou Medical University | March 1, 2022 | Not Applicable |
| NCT06010277 | Recruiting | Drug: Folinic acid | NSCLC Thymoma |
Amphia Hospital | February 6, 2023 | Phase 4 |
| NCT03837977 | Active Recruiting |
Drug: Folinic Acid Drug: Docetaxel |
Neuroendocrine Carcinoma Oncology |
The Christie NHS Foundation Trust | November 13, 2018 | Phase 2 |