| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
据信,乙磺酰米特引起的低电压激活的T钙通道的扩张是全身失活的原因。当将乙硫胺处理的 tau 细胞爆破蠕虫与载体对照进行比较时,总 tau 水平并未降低。因此,相对于总 Tau 水平的可溶性和不溶性(RIPA 可裂解)Tau 的定量证明了乙磺酰米的救援作用,以及与未处理的蠕虫相比,乙磺酰米处理的蠕虫中存在不正确折叠的肽。可溶性 Tau 的增加与不溶性 Tau 的显着减少成比例 [1]。 1 μM 剂量的乙亚胺不仅比 2 μM 或更高剂量的乙亚胺更有效,而且乙亚胺还会引起细胞毒性。 GABA 染色的免疫荧光表明,乙噻胺处理后,神经元中的 GABA 浓度分别在 0.1 和 1 μM 时突然增加。乙噻胺诱导核增殖后两到三天,可见 BrdU 染色。 Brdu染色后,高浓度核溶剂为25.27±0.48,而低浓度乙酰亚胺核切割剂为15.98±0.41。氯化锂 [2] 的该值为 11.05±0.2。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Bioavailability following oral administration is 93%. Ethosuximide is absorbed from the GI tract. Following oral administration of a single dose, peak blood concentrations are reached within 4 hours; however, about 4-7 days of therapy at usual dosage are required to achieve steady-state plasma concentrations. The plasma concentration required for therapeutic effect is generally considered to range from 40-100 ug/mL; plasma concentrations less than 40 ug/mL are rarely effective. The relationship between plasma ethosuximide concentrations and toxic effects of the drug has not been clearly established; however, plasma concentrations as high as 150 ug/mL have not been associated with signs of toxicity. Absorption of ethosuximide appears to be complete, and peak concentrations occur in plasma within about 3 hr after a single oral dose. Ethosuximide is not significantly bound to plasma proteins; during long-term therapy, the concentration in the CSF is similar to that in plasma. The apparent volume of distribution averages 0.7 L/kg. In vitro data suggest that there is no substantial degree of protein binding for ethosuximide. In one study in children, peak CSF concentrations of 25-50 ug/mL were achieved within 1-2 hours following a single 250-mg dose of ethosuximide. These concentrations were maintained for 12-24 hours, and the drug was still detectable in the CSF 65 hours after the drug was given. Ethosuximide is excreted slowly in urine. Approximately 20% of a dose is excreted unchanged and up to 50% may be excreted in urine as the hydroxylated metabolite and/or its glucuronide. Small amounts of unchanged drug are also excreted in bile and feces. For more Absorption, Distribution and Excretion (Complete) data for ETHOSUXIMIDE (9 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic, via CYP3A4 and CYP2E1. ... Metabolized by hepatic microsomal enzymes. In rats, ethosuximide ... is metabolized into monohydroxyethosuximides, 2-ethyl-3-hydroxy-2-methyl-succinimide ... stereoisomeric 2-(1-hydroxyethyl)-2-methylsuccinimides & ... 2-(2-hydrox yethyl)-2-methylsuccinimide ... which are excreted, in urine, in free form and as ether glucuronides. Different plasma profiles were obtained following admin of ethosuximide...to rat & man... Unchanged drug & only trace amt of metabolites were detected in rat plasma. In human plasma, diastereoisomers of 2-(1-hydroxyethyl)-2-methylsuccinimide...were major components. Ethosuximide is a chiral drug substance primarily indicated for the treatment of absence seizures. This drug is used clinically as the racemate. The human urinary metabolites of ethosuximide (I) have been studied using chiral gas chromatography (GC) and gas chromatography/mass spectrometry (GC/MS). The metabolites identified were the previously reported unchanged ethosuximide (I) enantiomers, all four stereoisomers of 2-(1-hydroxyethyl)-2-methylsuccinimide (II), and the four stereoisomers of 2-ethyl-3-hydroxy-2-methylsuccinimide (III). Through chemical derivatization methodology and GC/MS two enantiomers of a previously unreported metabolite of ethosuximide, 2-ethyl-2-hydroxymethylsuccinimide (VI), have been identified. Hepatic, via CYP3A4 and CYP2E1. Half Life: 53 hours Biological Half-Life 53 hours A total of 10 epileptic mothers treated with ethosuximide (ES) as well as their 13 newborns were included in this study. At birth fetal/maternal serum concentration ratios were 0.97 +/- 0.02 (n = 7) and ES half-lives in three neonates were 32, 37 and 38 hr. ... The plasma half-life of ethosuximide is about 60 hours in adults and about 30 hours in children. A total of 10 epileptic mothers treated with ethosuximide (ES) as well as their 13 newborns were included in this study. At birth fetal/maternal serum concentration ratios were 0.97 +/- 0.02 (n = 7) and ES half-lives in three neonates were 32, 37 and 38 hr. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Binds to T-type voltage sensitive calcium channels. Voltage-sensitive calcium channels (VSCC) mediate the entry of calcium ions into excitable cells and are also involved in a variety of calcium-dependent processes, including muscle contraction, hormone or neurotransmitter release, gene expression, cell motility, cell division and cell death. The isoform alpha-1G gives rise to T-type calcium currents. T-type calcium channels belong to the "low-voltage activated (LVA)" group and are strongly blocked by mibefradil. A particularity of this type of channels is an opening at quite negative potentials and a voltage-dependent inactivation. T-type channels serve pacemaking functions in both central neurons and cardiac nodal cells and support calcium signaling in secretory cells and vascular smooth muscle. They may also be involved in the modulation of firing patterns of neurons which is important for information processing as well as in cell growth processes. Hepatotoxicity Prospective studies suggest that chronic ethosuximide therapy is not accompanied by significant elevations in serum aminotransferase levels, but can increase gamma glutamyltranspeptidase levels. Clinically apparent hepatotoxicity from ethosuximide is very rare with few case reports published despite use of this agent for half a century. Futhermore, the liver injury in reported cases was usually mild and asymptomatic and a part of a generalized hypersensitivity syndrome with fever, rash, facial edema, lymphadenopathy, and eosinophilia or atypical lymphocytosis. The usual latency to onset of the hypersensitivity syndrome is 2 to 8 weeks. The typical serum enzyme elevations are a mixed-cholestatic-hepatocellular pattern and reported cases have not been jaundiced. While the product labeling for ethosuximide warns of hepatic dysfunction and recommends periodic monitoring of liver tests, clinically apparent liver injury with jaundice from ethosuximide is rare. Likelihood score: E* (suspected but unproven cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Average ethosuximide dosages of 50 to 60% of the maternal weight-adjusted dosage are excreted in human milk and infant plasma levels of 25 to 30% of maternal levels are common. Although no adverse effects attributable solely to ethosuximide in breastmilk have been reported, monitor the infant for drowsiness, adequate weight gain, and developmental milestones, especially in younger, exclusively breastfed infants and when using combinations of anticonvulsants. Measurement of an infant serum level might help rule out toxicity if there is a concern. ◉ Effects in Breastfed Infants An infant whose mother was taking ethosuximide 250 mg daily began exclusive breastfeeding on day 2 postpartum and continued through 4.5 months of observation. The infant developed normally during this time and had no signs of an adverse reaction. Sedation, poor sucking and poor weight gain during the first 4 weeks of life occurred in a breastfed newborn whose mother was taking ethosuximide. The reaction was possibly caused by ethosuximide in breastmilk; however, the mother was also taking primidone and valproic acid. Three fully breastfed infants and a mostly formula-fed whose mothers were taking ethosuximide had no adverse reactions observed during the first 1.5 to 4.5 months of life. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Interactions ... It is thus assumed that the probable mechanism of action of ethosuximide consists in lowering calcium transport since the inhibitors of calcium transport sodium nitroprusside and verapamil intensify the blocking effect of ethosuximide on smooth muscle contractile activity. Concurrent use /with alcohol; central nervous system depression-producing medications; tricyclic antidepressants; loxapine; maprotiline; molindone; monoamine oxidase inhibitors; phenothiazines; pimozide; thioxanthenes/ may lower the convulsive threshold, enhance CNS depression, and decrease the effects of the anticonvulsant medication. /Succinimide anticonvulsants/ Requirements for folic acid may be increased in patients receiving anticonvulsant therapy. /Succinimide anticonvulsants/ Induction of hepatic microsomal enzyme activity resulting in increased metabolism and decreased serum concentrations and elimination half-lives of succinimide anticonvulsants and/or these medications /carbamazepine, phenobarbital, phenytoin, primidone/ may occur during concurrent therapy; monitoring of serum concentrations as a guide to dosage is recommended, especially when any anticonvulsant is added to or withdrawn from an existing regimen. /Succinimide anticonvulsants/ For more Interactions (Complete) data for ETHOSUXIMIDE (8 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mouse iv 780 mg/kg LD50 Mouse sc 1810 mg/kg LD50 Mouse ip 1752 mg/kg LD50 Mouse oral 1530 mg/kg |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Anticonvulsants Ethosuximide, the drug of choice, and phensuximide are indicated for the control of seizures in absence (petit mal) epilepsy. /Included in US product labeling/ Drug Warnings Hemodialysis patients concurrently receiving ethosuximide may require a supplemental dose or an altered dosing schedule, based on the conclusion that ethosuximide is dialyzable. The most common dose-related side effects are gastrointestinal complaints (nausea, vomiting, and anorexia) and CNS effects (drowsiness, lethargy, euphoria, dizziness, headache, and hiccough). Some tolerance to these effects develops. Parkinsonlike symptoms and photophobia also have been reported. Restlessness, agitation, anxiety, aggressiveness, inability to concentrate, and other behavioral effects have occurred primarily in patients with a prior history of psychiatric disturbance. Urticaria and other skin reactions, including Stevens-Johnson syndrome, as well as systemic lupus erythematosus, eosinophilia, leukopenia, thrombocytopenia, pancytopenia, and aplastic anemia also have been attributed to the drug. The leukopenia may be transient, despite continuation of the drug, but several deaths have resulted from bone-marrow depression. Renal or hepatic toxicity has not been reported. The most common adverse effects of ethosuximide are GI symptoms including anorexia and weight loss, vague gastric upset, cramps, abdominal pain, diarrhea, nausea, vomiting, and epigastric distress. For more Drug Warnings (Complete) data for ETHOSUXIMIDE (13 total), please visit the HSDB record page. Pharmacodynamics Used in the treatment of epilepsy. Ethosuximide suppresses the paroxysmal three cycle per second spike and wave activity associated with lapses of consciousness which is common in absence (petit mal) seizures. The frequency of epileptiform attacks is reduced, apparently by depression of the motor cortex and elevation of the threshold of the central nervous system to convulsive stimuli. |
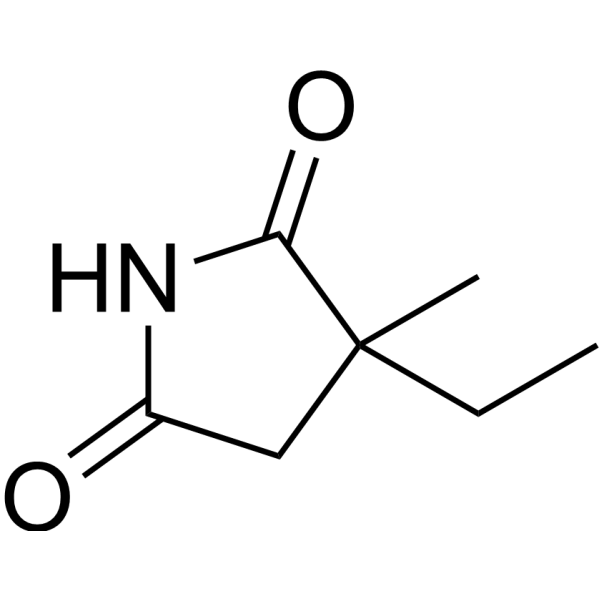
| 分子式 |
C7H11NO2
|
|---|---|
| 分子量 |
141.1677
|
| 精确质量 |
141.078
|
| CAS号 |
77-67-8
|
| 相关CAS号 |
Ethosuximide-d3;1189703-33-0;Ethosuximide-d5;1989660-59-4
|
| PubChem CID |
3291
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
265.3±9.0 °C at 760 mmHg
|
| 熔点 |
51ºC
|
| 闪点 |
123.8±18.9 °C
|
| 蒸汽压 |
0.0±0.5 mmHg at 25°C
|
| 折射率 |
1.451
|
| LogP |
0.38
|
| tPSA |
46.17
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
10
|
| 分子复杂度/Complexity |
188
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
HAPOVYFOVVWLRS-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C7H11NO2/c1-3-7(2)4-5(9)8-6(7)10/h3-4H2,1-2H3,(H,8,9,10)
|
| 化学名 |
3-ethyl-3-methylpyrrolidine-2,5-dione
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~708.37 mM)
H2O : ~100 mg/mL (~708.37 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (14.73 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (14.73 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (14.73 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 7.0837 mL | 35.4183 mL | 70.8366 mL | |
| 5 mM | 1.4167 mL | 7.0837 mL | 14.1673 mL | |
| 10 mM | 0.7084 mL | 3.5418 mL | 7.0837 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。