| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
体外活性:乙炔雌二醇可增加培养的大鼠肝细胞和 HepG2 细胞的呼吸链活性。乙炔雌二醇是肝癌发生的强促进剂。 Ethinyl Estradiol 增强雌性大鼠肝脏中核基因组和线粒体基因组编码基因的转录水平以及呼吸链活性,并且还抑制雌性大鼠培养的肝切片和肝细胞中转化生长因子β (TGFbeta) 诱导的细胞凋亡。乙炔雌二醇可增加培养的雌性大鼠细胞中线粒体基因组编码基因细胞色素氧化酶亚基 I、II 和 III 的转录水平。乙炔雌二醇显着增加线粒体和细胞核中每毫克蛋白质的谷胱甘肽(还原型 [GSH] 和氧化 [GSSG] 形式)水平,而氧化形式的总谷胱甘肽百分比不受影响。
|
||
|---|---|---|---|
| 体内研究 (In Vivo) |
乙炔雌二醇(50 毫克/公斤/天)会增加肛门生殖器距离,降低出生后第 2 天的幼犬体重,加速阴道开口年龄,降低 F1 生育力和 F2 窝产仔数,并诱发外生殖器畸形(5 毫克/公斤) )在雌性 Long-Evans 大鼠中。乙炔雌二醇会增加大鼠肝脏中低密度脂蛋白 (LDL) 受体的数量,从而显着降低血浆胆固醇水平。乙炔雌二醇在雄性和雌性兔子的肝脏中发挥相同的作用,并且受体数量的增加与受体 mRNA 水平的 6 至 8 倍增加相关。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
A 30µg oral dose of ethinylestradiol reaches a Cmax of 74.1±35.6pg/mL, with a Tmax of 1.5±0.5h, and an AUC of 487.4±166.6pg\*h/mL. A 1.2mg dose delivered via a patch reaches a Cmax of 28.8±10.3pg/mL, with a Tmax of 86±31h, and an AUC of3895±1423pg\*h/mL. Ethinylestradiol is 59.2% eliminated in the urine and bile, while 2-3% is eliminated in the feces. Over 90% of ethinylestradiol is eliminated as the unchanged parent drug. A 30µg oral dose has an apparent volume of distribution of 625.3±228.7L and a 1.2mg topical dose has an apparent volume of distribution of 11745.3±15934.8L. Ethinylestradiol has an intravenous clearance of 16.47L/h, and an estimated renal clearance of approximately 2.1L/h. A 30µg oral dose has a clearance of 58.0±19.8L/h and a 1.2mg topical dose has a clearance of 303.5±100.5L/h. Ethinyl estradiol is rapidly and almost completely absorbed. When the lowest and highest tablet strengths, 0.100 mg desogestrel/0.025 mg ethinyl estradiol and 0.150 mg desogestrel/0.025 mg ethinyl estradiol, were compared to solution, the relative bioavailability of ethinyl estradiol was 92% and 98%, respectively. The distribution of exogenous estrogens is similar to that of endogenous estrogens. Estrogens are widely distributed in the body and are generally found in higher concentrations in the sex hormone target organs. Ethinyl estradiol circulates in the blood largely bound to ... albumin. ... Although ethinyl estradiol does not bind to SHBG, it induces SHBG synthesis. Estradiol, estrone, and estriol are excreted in the urine along with glucuronide and sulfate conjugates. 25 healthy women of reproductive age who had not previously used oral contraceptive steroids, were each given a single tablet containing 50, 80, or 100 ug of mestranol or 50 or 80 ug of ethinyl estradiol. Blood samples were obtained before taking the tablets and at intervals of 1, 2, 4, and 24 hours afterward. Anti-ethinyl-estradiol antibody in 1 to 100,000 initial dilution was used. Details of techniques employed are given. With ethinyl estradiol, the 1-hour sampling yielded the maximum plasma levels. At 24 hours, the plasma level was not detectable in 4 of 5 subjects given 50 ug or in 1 of 5 given 80 ug. With mestranol, the disappearance curve was more variable with the peak levels usually at 2 hours but occasionally at 4 hours. At all 3 dose levels of mestranol, measurable serum ethinyl estradiol levels were found at 24 hours. These levels were reached more slowly and were lower than when ethinyl estradiol was given. In contrast to natural estrogens ethinyl, estrogens are bound to plasma proteins chiefly by nonspecific binding and are therefore less likely to affect the metabolism of the ethinyl estrogens than are the endogenous steroids. Also, significant amounts of ethinyl estradiol are was given. In contrast to natural estrogens ethinyl, estrogens are bound di-ethynylated in vitro. The pharmacokinetics of ethinyl estrogens differ from those of natural estrogens. This complicates interpretation of plasma or urinary estrone and estradiol measurements. For more Absorption, Distribution and Excretion (Complete) data for ETHINYLESTRADIOL (7 total), please visit the HSDB record page. Metabolism / Metabolites Ethinylestradiol can be glucuronidated by UGT1A1, UGT1A3, UGT1A4, UGT1A9, and UGT2B7. Ethinylestradiol is also sulfated by SULT1A1, SULT1A3, and SULT1E1. Ethinylestradiol can also be hydroxylated at positions 2, 4, 6, 7, and 16 by CYP3A4, CYP3A5, CYP2C8, CYP2C9, and CYP1A2. These hydroxylated metabolites can be methylated by catechol-O-methyltransferase. The methoxy metabolites can in turn be sulfated or glucuronidated. Exogenous estrogens are metabolized in the same manner as endogenous estrogens. Circulating estrogens exist in a dynamic equilibrium of metabolic interconversions. These transformations take place mainly in the liver. Estradiol is converted reversibly to estrone, and both can be converted to estriol, which is the major urinary metabolite. Estrogens also undergo enterohepatic recirculation via sulfate and glucuronide conjugation in the liver, biliary secretion of conjugates into the intestine, and hydrolysis in the gut followed by reabsorption. In postmenopausal women, a significant proportion of the circulating estrogens exist as sulfate conjugates, especially estrone sulfate, which serves as a circulating reservoir for the formation of more active estrogens. Ethinyl estradiol is extensively metabolized, both by oxidation and by conjugation with sulfate and glucuronide. Sulfates are the major circulating conjugates of ethinyl estradiol and glucuronides predominate in urine. The primary oxidative metabolite is 2-hydroxy ethinyl estradiol, formed by the CYP3A4 isoform of cytochrome P450. Part of the first-pass metabolism of ethinyl estradiol is believed to occur in gastrointestinal mucosa. Ethinyl estradiol may undergo enterohepatic circulation. Ethinyl estradiol is cleared much more slowly ... due to decreased hepatic metabolism. Studies on the metabolism of ethinylestradiol have been carried out in rats, rabbits, guinea-pigs, dogs and monkeys. It is very rapidly and effectively absorbed from rat intestine; no appreciable metabolic transformation is reported to take place during the absorption process. The main metabolic pathway of ethinylestradiol in rats is by aromatic 2-hydroxylation; hydroxylations at ring B (C-6/C-7) are of only minor importance. Rat liver forms 2-hydroxyethinylestradiol and the methyl ethers thereof, 2-methoxyethinylestradiol and 2-hydroxyethinylestradiol-3-methy1 ether, as its major metabolic products. This pathway is also important in humans. Metabolites of ethinylestradiol in rats are excreted almost exclusively in the feces. For more Metabolism/Metabolites (Complete) data for ETHINYLESTRADIOL (10 total), please visit the HSDB record page. Ethinylestradiol has known human metabolites that include 17-Ethynyl-13-methyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthrene-3,4,17-triol and 17-Ethynyl-13-methyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthrene-2,3,17-triol. Ethinylestradiol is a known human metabolite of Mestranol. Hepatic. Quantitatively, the major metabolic pathway for ethinyl estradiol, both in rats and in humans, is aromatic hydroxylation, as it is for the natural estrogens. Half Life: 36 +/- 13 hours Biological Half-Life A 30µg oral dose has a half life of 8.4±4.8h and a 1.2mg topical dose has a half life of 27.7±34.2h. The pharmacokinetics of 19-nor-17 alpha-pregna-1,3,5(10)-trien-20-yne-3,17-diol (ethinylestradiol, Progynon C) (EE2) has been studied after intravenous administration of 0.1 or 0.01 mg/kg and after intragastric administration of 1 mg/kg in female rats, rabbits, beagle dogs, rhesus monkeys and baboons. After intravenous administration disposition of unchanged drug in the plasma was biphasic with initial half-lives between 0.3 and 0.5 hr and terminal half-lives between 2.3 and 3.0 hr. Total plasma clearance was of the same magnitude as total plasma liver flow or even higher rat) indicating a rapid biotransformation of the estrogen in the liver. Systemic availability of intragastric EE2 amounted to 3% in the rat, 0.3% in the rabbit, 9% in the dog, 0.6% in rhesus monkeys and 2% in the baboon and was considerably lower than in humans (40%). Differences in the pharmacokinetics and in the systemic availability of EE2 between laboratory animals and man should be taken into account in the retrospective interpretation of pharmacological and toxicological data and in the design of new studies. ... The elimination phase half-life has been reported ... to be 13 to 27 hours. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation This record contains information specific to ethinyl estradiol used alone. Users with an interest in an oral contraceptive should consult the record entitled, record entitled, Contraceptives, Oral, Combined. There is little information available on the use of ethinyl estradiol alone during breastfeeding. Levels in milk appear to be low. Based on studies on oral contraceptives that contain ethinyl estradiol, immediate side effects such as breast enlargement appear to occur rarely. It seems likely that doses of 30 mcg daily or greater can suppress lactation. The magnitude of the effect on lactation likely depends on the dose and the time of introduction postpartum. It is most likely to occur if the estrogen is started before the milk supply is well established at about 6 weeks postpartum. The decrease can happen over the first few days of estrogen exposure. ◉ Effects in Breastfed Infants Published information was not found as of the revision date on the effects of ethinyl estradiol alone on breastfed infants. However, case reports exist of breast enlargement in the infants of mothers taking combination oral contraceptives that contained ethinyl estradiol or its prodrug, mestranol. ◉ Effects on Lactation and Breastmilk Published information was not found as of the revision date on the effects of ethinyl estradiol on milk production. However, numerous studies on combination contraceptives containing ethinyl estradiol or its prodrug mestranol indicate that doses of 30 mcg daily or greater might interfere with lactation. One study that used a contraceptive containing 10 mcg of ethinyl estradiol found no effect on lactation. A retrospective cohort study compared 371 women who received high-dose estrogen (either 3 mg of diethylstilbestrol or 150 mcg of ethinyl estradiol daily)during adolescence for adult height reduction to 409 women who did not receive estrogen. No difference in breastfeeding duration was found between the two groups, indicating that high-dose estrogen during adolescence has no effect on later breastfeeding. Protein Binding Enthinylestradiol is 98.3-98.5% bound to albumin in serum but also exhibits binding to sex hormone binding globulin. |
||
| 参考文献 | |||
| 其他信息 |
Ethinylestradiol can cause cancer according to an independent committee of scientific and health experts.
Ethinylestradiol is a fine white to creamy white powder. A synthetic steroid. Used in combination with progestogen as an oral contraceptive. 17alpha-ethynylestradiol is a 3-hydroxy steroid that is estradiol substituted by a ethynyl group at position 17. It is a xenoestrogen synthesized from estradiol and has been shown to exhibit high estrogenic potency on oral administration. It has a role as a xenoestrogen. It is a 17-hydroxy steroid, a terminal acetylenic compound and a 3-hydroxy steroid. It is functionally related to a 17beta-estradiol and an estradiol. Ethinylestradiol was first synthesized in 1938 by Hans Herloff Inhoffen and Walter Hohlweg at Schering. It was developed in an effort to create an estrogen with greater oral bioavailability. These properties were achieved by the substitution of an ethinyl group at carbon 17 of [estradiol]. Ethinylestradiol soon replaced [mestranol] in contraceptive pills. Ethinylestradiol was granted FDA approval on 25 June 1943. Ethinyl estradiol is an Estrogen. The mechanism of action of ethinyl estradiol is as an Estrogen Receptor Agonist. Ethinyl estradiol has been reported in Minthostachys mollis, Elsholtzia eriostachya, and other organisms with data available. Ethinyl Estradiol is a semisynthetic estrogen. Ethinyl estradiol binds to the estrogen receptor complex and enters the nucleus, activating DNA transcription of genes involved in estrogenic cellular responses. This agent also inhibits 5-alpha reductase in epididymal tissue, which lowers testosterone levels and may delay progression of prostatic cancer. In addition to its antineoplastic effects, ethinyl estradiol protects against osteoporosis. In animal models, short-term therapy with this agent has been shown to provide long-term protection against breast cancer, mimicking the antitumor effects of pregnancy. (NCI04) A semisynthetic alkylated estradiol with a 17-alpha-ethinyl substitution. It has high estrogenic potency when administered orally and is often used as the estrogenic component in oral contraceptives . Ethinyl estradiol is marketed mostly as a combination oral contraceptive under several brand names such as Alesse, Tri-Cyclen, Triphasil, and Yasmin. The FDA label includes a black box warning that states that combination oral contraceptives should not be used in women over 35 years old who smoke due to the increased risk of serious cardiovascular side effects. A semisynthetic alkylated ESTRADIOL with a 17-alpha-ethinyl substitution. It has high estrogenic potency when administered orally, and is often used as the estrogenic component in ORAL CONTRACEPTIVES. See also: Ethinyl estradiol; norgestrel (component of); Ethinyl estradiol; ethynodiol diacetate (component of); Ethinyl estradiol; etonogestrel (component of) ... View More ... Drug Indication Ethinylestradiol is combined with other drugs for use as a contraceptive, premenstrual dysphoric disorder, moderate acne, moderate to severe vasomotor symptoms of menopause, prevention of postmenopausal osteoporosis. FDA Label Mechanism of Action Ethinylestradiol is a synthetic estrogenic compound. Use of estrogens have a number of effects on the body including reduced bone density. Combined oral contraceptives suppress ovulation by suppressing gonadotrophic hormone, thickening cervical mucus to prevent the travel of sperm, and preventing changes in the endometrium required for implantation of a fertilized egg. Ethinylestradiol decreases luteinizing hormone, decreasing vascularity in the endometrium. It also increases sex hormone binding globulin. Endogenous estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Although circulating estrogens exist in a dynamic equilibrium of metabolic interconversions, estradiol is the principal intracellular human estrogen and is substantially more potent than its metabolites estrone and estriol at the receptor level. ... After menopause, most endogenous estrogen is produced by conversion of androstenedione, secreted by the adrenal cortex, to estrone by peripheral tissues. Thus, estrone and the sulfate conjugated form, estrone sulfate, are the most abundant circulating estrogens in postmenopausal women. The pharmacologic effects of ethinyl estradiol are similar to those of endogenous estrogens. Estrogens act through binding to nuclear receptors in estrogen-responsive tissues. To date, two estrogen receptors have been identified. These vary in proportion from tissue to tissue. Circulating estrogens modulate the pituitary secretion of the gonadotropins, luteinizing hormone (LH) and follicle stimulating hormone (FSH) through a negative feedback mechanism. Estrogens act to reduce the elevated levels of these hormones seen in postmenopausal women. Estrogens have an important role in the reproductive, skeletal, cardiovascular, and central nervous systems in women, and act principally by regulating gene expression. Biologic response is initiated when estrogen binds to a ligand-binding domain of the estrogen receptor resulting in a conformational change that leads to gene transcription through specific estrogen response elements (ERE) of target gene promoters; subsequent activation or repression of the target gene is mediated through 2 distinct transactivation domains (ie, AF-1 and AF-2) of the receptor. The estrogen receptor also mediates gene transcription using different response elements (ie, AP-1) and other signal pathways. Recent advances in the molecular pharmacology of estrogen and estrogen receptors have resulted in the development of selective estrogen receptor modulators (eg, clomiphene, raloxifene, tamoxifen, toremifene), agents that bind and activate the estrogen receptor but that exhibit tissue-specific effects distinct from estrogen. Tissue-specific estrogen-agonist or -antagonist activity of these drugs appears to be related to structural differences in their estrogen receptor complex (eg, specifically the surface topography of AF-2 for raloxifene) compared with the estrogen (estradiol)-estrogen receptor complex. A second estrogen receptor also has been identified, and existence of at least 2 estrogen receptors (ER-alpha, ER-beta) may contribute to the tissue-specific activity of selective modulators. While the role of the estrogen receptor in bone, cardiovascular tissue, and the CNS continues to be studied, emerging evidence indicates that the mechanism of action of estrogen receptors in these tissues differs from the manner in which estrogen receptors function in reproductive tissue. /Estrogen General Statement/ Intracellular cytosol-binding proteins for estrogens have been identified in estrogen-responsive tissues including the female genital organs, breasts, pituitary, and hypothalamus. The estrogen-binding protein complex (ie, cytosol-binding protein and estrogen) distributes into the cell nucleus where it stimulates DNA, RNA, and protein synthesis. The presence of these receptor proteins is responsible for the palliative response to estrogen therapy in women with metastatic carcinoma of the breast. /Estrogen General Statement/ Estrogens have generally favorable effects on blood cholesterol and phospholipid concentrations. Estrogens reduce LDL-cholesterol and increase HDL-cholesterol concentrations in a dose-related manner. The decrease in LDL-cholesterol concentrations associated with estrogen therapy appears to result from increased LDL catabolism, while the increase in triglyceride concentrations is caused by increased production of large, triglyceride-rich, very-low-density lipoproteins (VLDLs); changes in serum HDL-cholesterol concentrations appear to result principally from an increase in the cholesterol and apolipoprotein A-1 content of HDL2- and a slight increase in HDL3-cholesterol. /Estrogen General Statement/ For more Mechanism of Action (Complete) data for ETHINYLESTRADIOL (7 total), please visit the HSDB record page. |
| 分子式 |
C20H24O2
|
|
|---|---|---|
| 分子量 |
296.4
|
|
| 精确质量 |
296.177
|
|
| CAS号 |
57-63-6
|
|
| 相关CAS号 |
|
|
| PubChem CID |
5991
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
457.2±45.0 °C at 760 mmHg
|
|
| 熔点 |
182-183 °C(lit.)
|
|
| 闪点 |
211.2±23.3 °C
|
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
|
| 折射率 |
1.624
|
|
| LogP |
4.52
|
|
| tPSA |
40.46
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
2
|
|
| 可旋转键数目(RBC) |
1
|
|
| 重原子数目 |
22
|
|
| 分子复杂度/Complexity |
505
|
|
| 定义原子立体中心数目 |
5
|
|
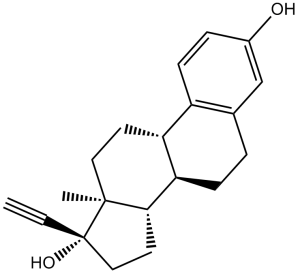
| SMILES |
C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@]2(C#C)O)CCC4=C3C=CC(=C4)O
|
|
| InChi Key |
BFPYWIDHMRZLRN-SLHNCBLASA-N
|
|
| InChi Code |
InChI=1S/C20H24O2/c1-3-20(22)11-9-18-17-6-4-13-12-14(21)5-7-15(13)16(17)8-10-19(18,20)2/h1,5,7,12,16-18,21-22H,4,6,8-11H2,2H3/t16-,17-,18+,19+,20+/m1/s1
|
|
| 化学名 |
(8R,9S,13S,14S,17R)-17-ethynyl-13-methyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthrene-3,17-diol
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.3738 mL | 16.8691 mL | 33.7382 mL | |
| 5 mM | 0.6748 mL | 3.3738 mL | 6.7476 mL | |
| 10 mM | 0.3374 mL | 1.6869 mL | 3.3738 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。