| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
Caspase; caspase-3
|
|---|---|
| 体外研究 (In Vitro) |
Emricasan,也称为 IDN-6556 或 PF-03491390,是一种亚至纳摩尔活性半胱天冬酶抑制剂。虽然 Emricasan 对 hNPC 具有神经保护活性,但并不能阻止 ZIKV 复制[2]。
Emricasan,一种泛caspase抑制剂,被鉴定为最有效的抗死亡化合物,在SNB-19细胞对抗三种ZIKV菌株:MR766(1947年乌干达菌株),FSS13025(2010年柬埔寨菌株)和PRVABC59(2015年波多黎各菌株)的caspase活性和细胞活力测试中,IC50值为0.13 - 0.9 μM(图1a)。此外,Emricasan在单层和3D类器官培养中减少了FSS13025感染的活性(裂解的)表达caspase-3的前脑特异性hNPCs的数量(图1b-c)。与模拟处理相比,Emricasan处理暴露于zikv的脑类器官似乎没有影响hNPC的增殖,通过磷酸化组蛋白3 (PH3)表达来评估(106±10%;N = 8;P = 0.7;单向方差分析)。值得注意的是,在Emricasan处理后,寨卡病毒抗原在2D和3D培养物中都存在(图1b-c)。因此,Emricasan对hNPCs表现出神经保护活性,但不抑制ZIKV复制。[2] |
| 体内研究 (In Vivo) |
Emricasan 可减少 NASH 相关的肝损伤,但不会减少代谢紊乱。它还可以减轻炎症。泛半胱天冬酶抑制剂 Emricasan 可减少小鼠 NASH 模型中的肝纤维形成和星状细胞活化[1]。 emricasan 目前正在进行 2 期临床试验,以确定它是否可以减轻慢性 HCV 感染引起的肝损伤和肝纤维化[2]。
TUNEL实验显示,饲喂HFD的小鼠肝细胞凋亡增加了5倍,caspase-3和8的活性分别增加了1.5倍和1.3倍;在饲喂Emricasan (HFD- em)处理的HFD的小鼠中,这种细胞凋亡的增加明显减弱。同样,通过测定血清天冬氨酸转氨酶和丙氨酸转氨酶水平、NAS组织学评分和IL -1 -β、TNF-α、单核细胞趋化蛋白(MCP-1)和C-X-C趋化因子配体-2 (CXCL2)定量反转录聚合酶链反应(qPCR),与HFD相比,饲喂HFD- em小鼠的肝损伤和炎症减轻。这些差异不能归因于肝脂肪变性的差异,因为两个HFD组的肝脏甘油三酯含量相似。Emricasan通过qPCR降低α - sma(肝星状细胞活化标志)、纤维化评分、天狼星红染色、羟脯氨酸肝含量和促纤维化细胞因子,减少HFD动物的肝纤维化。 结论:总之,这些数据表明,在NASH小鼠模型中,肝损伤和纤维化通过抑制肝细胞凋亡而受到抑制,这表明Emricasan可能是一种有吸引力的NASH抗纤维化治疗药物。[2] |
| 酶活实验 |
通过定量高通量筛选(qHTS),每种化合物分别在4种浓度(0.37、1.84、9.2和46 μM)下进行单通道筛选。虽然单一化合物浓度(单线态)传统上用于大型化合物集合(如100万至300万化合物)的HTS,但具有多种化合物浓度的qHTS格式最近已用于中型或小型化合物集合,如批准的药物文库。具体地说,将SNB-19细胞和hNPCs以每3 μl/孔250个细胞的速度接种到PDL包被的1536孔实验板上,在37℃、5% CO2中孵育16小时。溶解在DMSO中的测试化合物通过自动pintool工作站以23 nl/孔的体积转移到分析板上。化合物与细胞在37℃、5% CO2中孵育30分钟,随后立即加入2 μl/孔的ZIKV (2 FFU/细胞)。寨卡病毒复合处理细胞的孵育时间因实验格式不同而不同。测量病毒诱导的caspase-3/7活性的实验需要将ZIKV在37°C、5% CO2中存在化合物的条件下孵育6小时。孵育后,在检测板上加入3.5 μl/孔的caspase-3/7混合试剂。在室温下孵育30分钟,用ViewLux平板阅读器测量产生的发光信号。测量病毒诱导的细胞死亡的实验需要将寨卡病毒在化合物存在下,在37°C、5% CO2中孵育72小时。孵育后,在测定板上加入3.5 μl/孔的ATP含量检测试剂。在室温下孵育30分钟,在ViewLux平板阅读器上测量产生的发光信号。逐步测定方案列在补充表1和2中。[2]
|
| 细胞实验 |
星形胶质细胞被模拟感染,用 DMSO 处理,或用 2 M 氯硝柳胺、92 M PHA-690509、9 μM emricasan 或 92 μM PHA-690509 和 9 M emricasan 的组合处理 1 小时,然后用 PRVABC59 感染 (MOI) = 0.5)。感染 24 小时后,固定细胞并进行 ZIKVE 和细胞核染色。
ATP含量测定对细胞活力和复合细胞毒性的影响[2] 采用ATPlite发光检测系统检测试剂盒测定细胞活力。按照制造商的描述对试剂进行重组和制备。为测定寨卡病毒感染后的细胞死亡情况,将细胞置于37℃5% CO2培养皿中培养16 h,然后加入寨卡病毒培养液,37℃5% CO2孵育72 h。然后将ATP监测试剂ATPlite加入测定板中,孵育15分钟。使用ViewLux平板阅读器测量产生的发光。将没有细胞的孔作为100%细胞杀伤的对照,将没有寨卡病毒感染的细胞孔作为完全细胞存活率(0%细胞杀伤),将数据归一化。为了分析所选化合物的潜在毒性,将细胞接种于96孔板中。1天后,用指定的化合物和浓度处理细胞24-48小时,然后加入Cell滴度- glo底物,并根据制造商的说明进行测量。 |
| 动物实验 |
Reagents and formulation [1]
Emricasan (formerly named IDN-6556 or PF-03491390) was suspended in vehicle [2% (v/v) DMSO in 0.5% (w/v) methylcellulose] and administered to mice per os daily. The high fat diet (HFD) used was used, which contains 47% of calories from fat (mostly from Milk fat, 50% saturated fat) with 2% of cholesterol, 35% from carbohydrate (78% of carbohydrate from Sucrose) and 18% of calories from protein, and was designed to approximate the typical human diet from patients with NASH. Animals[1] Studies were performed in male C57BL/6J mice. All animals were maintained in a temperature (24°C) and light controlled (12:12 h light:dark) facility, and had free access to food and water. Animals were age-matched and used at approximately 12–16 weeks of age. Four groups were studied (n = 60) with 15 mice per group. Groups 1 and 3 received regular chow. Groups 2 and 4 received HFD and 50 g/L (Sucrose) was added to drinking water for 20 weeks. Groups 3 and 4 received Emricasan 0.3 mg/kg/day per os, and Group 1 and 2 received the vehicle. The dosing was based on previous data 21 that demonstrates that oral administration of Emricasan at doses of 0.3 mg/kg corresponded to the ED90 value to prevent liver injury in the model of α-Fas-induced liver injury. Total body weight was measured at 0, 5, 10, 15 and 20 weeks. |
| 参考文献 | |
| 其他信息 |
Emricasan is the first caspase inhibitor tested in human which has received orphan drug status by FDA. It is developed by Pfizer and made in such a way that it protects liver cells from excessive apoptosis.
Drug Indication Investigated for use/treatment in hepatitis (viral, C), liver disease, and transplantation (organ or tissue). Treatment of non-alcoholic steatohepatitis (NASH) Mechanism of Action IDN-6556 significantly improves markers of liver damage in patients infected with the Hepatitis C virus (HCV), an infection that affects up to 170m patients worldwide. IDN-6556 represents a new class of drugs that protect the liver from inflammation and cellular damage induced by viral infections and other causes. Various studies have also shown that it significantly lowers aminotransferase activity in HCV patients and appeared to be well tolerated. Background & aims: Hepatocyte apoptosis, the hallmark of non-alcoholic steatohepatitis (NASH) contributes to liver injury and fibrosis. Although, both the intrinsic and extrinsic apoptotic pathways are involved in the pathogenesis of NASH, the final common step of apoptosis is executed by a family of cysteine-proteases termed caspases. Thus, our aim was to ascertain if administration of Emricasan, a pan-caspase inhibitor, ameliorates liver injury and fibrosis in a murine model of NASH. Methods: C57/BL6J-mice were fed regular chow or high fat diet (HFD) for 20 weeks. All mice were treated with vehicle or Emricasan. Results: Mice fed a HFD diet demonstrate a five-fold increase in hepatocyte apoptosis by the TUNEL assay and a 1.5-fold and 1.3-fold increase in caspase-3 and-8 activities respectively; this increase in apoptosis was substantially attenuated in mice fed a HFD treated with Emricasan (HFD-Em). Likewise, liver injury and inflammation were reduced in mice fed HFD-Em as compare to HFD by measuring serum aspartate aminotransferase and alanine aminotransferase levels, NAS histological score and IL 1-β, TNF-α, monocyte chemoattractant protein (MCP-1) and C-X-C chemokine ligand-2 (CXCL2) quantitative reverse-transcription polymerase chain reaction (qPCR). These differences could not be attributed to differences in hepatic steatosis as liver triglycerides content were similar in both HFD groups. Hepatic fibrosis was reduced by Emricasan in HFD animals by decreasing αSMA (a marker for hepatic stellate cell activation), fibrosis score, Sirius red staining, hydroxyproline liver content and profibrogenic cytokines by qPCR. Conclusion: In conclusion, these data demonstrate that in a murine model of NASH, liver injury and fibrosis are suppressed by inhibiting hepatocytes apoptosis and suggests that Emricasan may be an attractive antifibrotic therapy in NASH.[1] In response to the current global health emergency posed by the Zika virus (ZIKV) outbreak and its link to microcephaly and other neurological conditions, we performed a drug repurposing screen of ∼6,000 compounds that included approved drugs, clinical trial drug candidates and pharmacologically active compounds; we identified compounds that either inhibit ZIKV infection or suppress infection-induced caspase-3 activity in different neural cells. A pan-caspase inhibitor, emricasan, inhibited ZIKV-induced increases in caspase-3 activity and protected human cortical neural progenitors in both monolayer and three-dimensional organoid cultures. Ten structurally unrelated inhibitors of cyclin-dependent kinases inhibited ZIKV replication. Niclosamide, a category B anthelmintic drug approved by the US Food and Drug Administration, also inhibited ZIKV replication. Finally, combination treatments using one compound from each category (neuroprotective and antiviral) further increased protection of human neural progenitors and astrocytes from ZIKV-induced cell death. Our results demonstrate the efficacy of this screening strategy and identify lead compounds for anti-ZIKV drug development.[2] |
| 分子式 |
C26H27F4N3O7
|
|---|---|
| 分子量 |
569.5021
|
| 精确质量 |
569.178
|
| 元素分析 |
C, 54.83; H, 4.78; F, 13.34; N, 7.38; O, 19.66
|
| CAS号 |
254750-02-2
|
| 相关CAS号 |
254750-02-2;
|
| PubChem CID |
12000240
|
| 外观&性状 |
white solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 折射率 |
1.550
|
| LogP |
4.63
|
| tPSA |
150.9
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
11
|
| 重原子数目 |
40
|
| 分子复杂度/Complexity |
934
|
| 定义原子立体中心数目 |
2
|
| SMILES |
FC1C(=C([H])C(=C(C=1OC([H])([H])C([C@]([H])(C([H])([H])C(=O)O[H])N([H])C([C@]([H])(C([H])([H])[H])N([H])C(C(N([H])C1=C([H])C([H])=C([H])C([H])=C1C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H])=O)=O)=O)=O)F)F)F
|
| InChi Key |
SCVHJVCATBPIHN-SJCJKPOMSA-N
|
| InChi Code |
InChI=1S/C26H27F4N3O7/c1-12(31-24(38)25(39)32-16-8-6-5-7-13(16)26(2,3)4)23(37)33-17(10-19(35)36)18(34)11-40-22-20(29)14(27)9-15(28)21(22)30/h5-9,12,17H,10-11H2,1-4H3,(H,31,38)(H,32,39)(H,33,37)(H,35,36)/t12-,17-/m0/s1
|
| 化学名 |
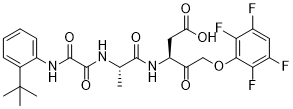
(3S)-3-[[(2S)-2-[[2-(2-tert-butylanilino)-2-oxoacetyl]amino]propanoyl]amino]-4-oxo-5-(2,3,5,6-tetrafluorophenoxy)pentanoic acid
|
| 别名 |
Emricasan; PF 03491390; PF-03491390; IDN-6556; (S)-3-((S)-2-(2-(2-TERT-BUTYLPHENYLAMINO)-2-OXOACETAMIDO)PROPANAMIDO)-4-OXO-5-(2,3,5,6-TETRAFLUOROPHENOXY)PENTANOIC ACID; (S)-3-((S)-2-(2-((2-(tert-Butyl)phenyl)amino)-2-oxoacetamido)propanamido)-4-oxo-5-(2,3,5,6-tetrafluorophenoxy)pentanoic acid; C26H27F4N3O7; PF03491390; IDN-6556; IDN6556; IDN 6556
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~100 mg/mL (~175.6 mM)
Ethanol: ~25 mg/mL (~43.9 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.5 mg/mL (4.39 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (4.39 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.39 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 5%DMSO+40%PEG300+5%Tween80+50%ddH2O: 5mg/ml 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7559 mL | 8.7796 mL | 17.5593 mL | |
| 5 mM | 0.3512 mL | 1.7559 mL | 3.5119 mL | |
| 10 mM | 0.1756 mL | 0.8780 mL | 1.7559 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02960204 | Completed | Drug: Emricasan Drug: Placebo |
Cirrhosis Portal Hypertension |
Histogen | October 17, 2016 | Phase 2 |
| NCT02230683 | Completed | Drug: IDN-6556 | Liver Cirrhosis | Conatus Pharmaceuticals Inc. | August 2014 | Phase 2 |
| NCT02686762 | Completed | Drug: Emricasan (5 mg) Drug: Emricasan (50 mg) |
Fibrosis Liver Diseases |
Conatus Pharmaceuticals Inc. | January 26, 2016 | Phase 2 |
| NCT04803227 | Terminated | Drug: Emricasan Other: Placebo |
Covid19 | Histogen | March 11, 2021 | Phase 1 |
|