| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 体内研究 (In Vivo) |
多巴酚丁胺起效快,半衰期短[2]。随着剂量的增加,多巴酚丁胺(0.15-20 mg/kg;腹腔注射)会导致野生型小鼠的左心室功能增强,心率增加[3]。高剂量的多巴酚丁胺在野生型小鼠中引起显着的正性肌力、舒张和变时性心脏反应[3]。低剂量多巴酚丁胺显着增强 Tgαq*44 动物的正性肌力和舒张心脏性能,而没有变时性改变 [3]。多巴酚丁胺仅在高剂量时才会增加心率,但随后会导致正性肌力和舒张心脏功能储备的丧失[3]。多巴酚丁胺通过激活 β-2 受体加速通气大鼠的肺泡液清除[4]。
|
|---|---|
| 动物实验 |
Animal/Disease Models: Tgαq*44 mouse (heart failure model) [3]
Doses: low dose 0.15mg/kg, 0.5mg/kg, high dose 1.5mg/kg, 5mg/kg, 20mg/kg: intraperitoneal (ip) injection Experimental Results:Low Low and high doses produced differential responses in cardiac function in mice with heart failure. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
In human urine, the major excretion products are the conjugates of dobutamine and 3-O-methyl dobutamine. Biological Half-Life 2 minutes |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No information is available on the use of dobutamine during breastfeeding. Because of its poor oral bioavailability and short half-life, any dobutamine in milk is unlikely to affect the infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information in nursing mothers was not found as of the revision date. Unlike dopamine, dobutamine infusion does not affect serum prolactin concentration in infants and in adult males. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. |
| 参考文献 |
[1]. Tuttle RR, et al. Dobutamine: development of a new catecholamine to selectively increase cardiac contractility. Circ Res. 1975 Jan;36(1):185-96.
[2]. Vallet B, et al. Dobutamine: mechanisms of action and use in acute cardiovascular pathology. Ann Cardiol Angeiol (Paris). 1991 Jun;40(6):397-402. [3]. Tyrankiewicz U , et al. Characterization of the cardiac response to a low and high dose of dobutamine in the mouse model of dilated cardiomyopathy by MRI in vivo. J Magn Reson Imaging. 2013 Mar;37(3):669-77. [4]. Tibayan FA, et al. Dobutamine increases alveolar liquid clearance in ventilated rats by beta-2 receptor stimulation. Am J Respir Crit Care Med. 1997 Aug;156(2 Pt 1):438-44. |
| 其他信息 |
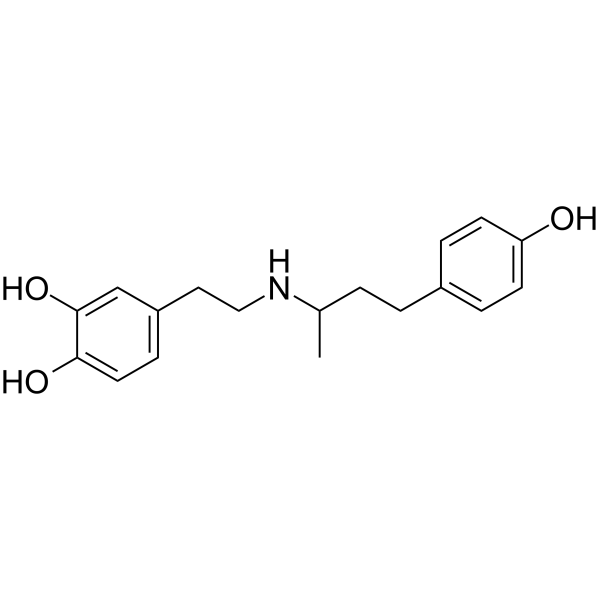
Dobutamine is a catecholamine that is 4-(3-aminobutyl)phenol in which one of the hydrogens attached to the nitrogen is substituted by a 2-(3,4-dihydroxyphenyl)ethyl group. A beta1-adrenergic receptor agonist that has cardiac stimulant action without evoking vasoconstriction or tachycardia, it is used as the hydrochloride to increase the contractility of the heart in the management of acute heart failure. It has a role as a cardiotonic drug, a sympathomimetic agent and a beta-adrenergic agonist. It is a secondary amine and a catecholamine.
A beta-1 agonist catecholamine that has cardiac stimulant action without evoking vasoconstriction or tachycardia. It is proposed as a cardiotonic after myocardial infarction or open heart surgery. Dobutamine is a beta-Adrenergic Agonist. The mechanism of action of dobutamine is as an Adrenergic beta-Agonist. Dobutamine is a synthetic catecholamine with sympathomimetic activity. Dobutamine is a direct-acting inotropic agent and an adrenergic agonist that stimulates primarily the beta-1 adrenoceptor, with lesser effect on beta-2 or alpha receptors. Via beta-1 adrenoceptor of the heart, this agent induces positive inotropic effect with minimal changes in chronotropic activities or systemic vascular resistance. Dobutamine also causes vasodilation by stimulating beta-2 adrenergic receptors in blood vessels, augmented by reflex vasoconstriction resulting in increased cardiac output. A catecholamine derivative with specificity for BETA-1 ADRENERGIC RECEPTORS. It is commonly used as a cardiotonic agent after CARDIAC SURGERY and during DOBUTAMINE STRESS ECHOCARDIOGRAPHY. See also: Dobutamine Hydrochloride (has salt form); Dobutamine Tartrate (has salt form); Dobutamine Lactobionate (is active moiety of). Drug Indication Indicated when parenteral therapy is necessary for inotropic support in the short-term treatment of patients with cardiac decompensation due to depressed contractility resulting either from organic heart disease or from cardiac surgical procedures. Treatment of neonatal circulatory failure Mechanism of Action Dobutamine directly stimulates beta-1 receptors of the heart to increase myocardial contractility and stroke volume, resulting in increased cardiac output. Pharmacodynamics Dobutamine is a direct-acting inotropic agent whose primary activity results from stimulation of the beta-adrenoceptors of the heart while producing comparatively mild chronotropic, hypertensive, arrhythmogenic, and vasodilative effects. Dobutamine acts primarily on beta-1 adrenergic receptors, with negligible effects on beta-2 or alpha receptors. It does not cause the release of endogenous norepinephrine, as does dopamine. |
| 分子式 |
C18H23NO3
|
|---|---|
| 精确质量 |
301.168
|
| CAS号 |
34368-04-2
|
| 相关CAS号 |
Dobutamine hydrochloride;49745-95-1;Dobutamine tartrate;101626-66-8
|
| PubChem CID |
36811
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.189g/cm3
|
| 沸点 |
527.7ºC at 760mmHg
|
| 熔点 |
184-186
|
| 闪点 |
169.8ºC
|
| LogP |
3.347
|
| tPSA |
72.72
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
22
|
| 分子复杂度/Complexity |
305
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CC(CCC1=CC=C(C=C1)O)NCCC2=CC(=C(C=C2)O)O
|
| InChi Key |
JRWZLRBJNMZMFE-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C18H23NO3/c1-13(2-3-14-4-7-16(20)8-5-14)19-11-10-15-6-9-17(21)18(22)12-15/h4-9,12-13,19-22H,2-3,10-11H2,1H3
|
| 化学名 |
4-[2-[4-(4-hydroxyphenyl)butan-2-ylamino]ethyl]benzene-1,2-diol
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Use of Dobutamine in Patients With Sepsis and Maintained Hypoperfusion After Initial Volemic Resuscitation.
CTID: NCT05953142
Phase: Phase 2 Status: Not yet recruiting
Date: 2024-01-30