| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|

| 靶点 |
Glucocorticoid receptor
|
|---|---|
| 体外研究 (In Vitro) |
地塞米松磷酸二钠通过调节许多调节因子(例如激活蛋白-1、核因子-AT 和核因子-kB)来促进参与调节反应的重要基因的激活和抑制[1]。地塞米松磷酸二钠的 EC50 为 2.2 nM,可有效控制 A549 细胞释放粒细胞-巨噬细胞集落刺激因子 (GM-CSF)。在浓度提高 10-100 倍时,地塞米松磷酸二钠 (EC50=36 nM) 通过感应 β2 红外受体并附着在葡萄糖信号接收激素 (GR) 的 DNA 上,抑制 GM-CSF 的释放。 GM-CSF 释放的抑制与地塞米松磷酸二钠 (IC50=0.5 nM) 对 3×κB(NF-κB、IκBα 和 I-κBβ)的抑制有关 [2]。
|
| 体内研究 (In Vivo) |
用 2 × 5 mg/kg 剂量的地塞米松磷酸二钠治疗成功抑制了脂多糖 (LPS) 诱导的受体。当小鼠暴露于脂多糖(LPS)并单次注射地塞米松磷酸二钠(10 mg/kg(ip))时,我们的实验系统中粒细胞募集和氧自由度均显着下降。革命者自发地涌现。吸入 LPS 前一小时和吸入后一小时,效果具有统计学意义。当向健康动物施用水雾剂时,BALF 中的粒细胞数量下降至与这些动物相似的值 [3]。与图表相比,用地塞米松磷酸二钠治疗的老鼠吃的食物更少,体重也更轻。尽管地塞米松磷酸二钠注射液的剂量在食品指南范围内,但五天的注射期导致肝脏质量(+42%)和肝脏相对体重(+65%)显着增加。治疗5天后,腓肠肌的肌肉是相同的,但与喂食的动物相比,动物的体重也有所减轻。湿重下降了 20%,但相对重量保持完全相同(克/100 克体重),表明手术后体重减轻与体重减轻相协调[4]。
|
| 酶活实验 |
1.糖皮质激素在控制哮喘和类风湿性关节炎等慢性炎症性疾病方面非常有效,但其抗炎作用的确切分子机制尚不清楚。它们通过与细胞质受体(GR)结合来激活或抑制基因表达。这可能是通过GR与DNA的直接结合(反式激活)或通过抑制AP-1和NF-kappaB等转录因子的活性(反式抑制)发生的。2.局部活性类固醇丙酸氟替卡松(EC50=1.8 x 10(-11)M)和布地奈德(EC50=5.0 x 10(-11)M)在抑制A549细胞释放GM-CSF方面比替普瑞定(EC50=8.3 x 10(-10)M)、布替西科特(EC50=3.7 x 10(-8)M)以及地塞米松(EC50=2.2 x 10(-9)M)更有效。抗糖皮质激素RU486还抑制了这些细胞中GM-CSF的释放(IC50=1.8 x 10(-10)M)。3.发现丙酸氟替卡松(EC50=9.8 x 10(-10)M)、布地奈德(EC50=1.1 x 10(-9)M)和地塞米松(EC50=3.6 x 10(-8)M)诱导β2受体转录的浓度依赖性能力与GR DNA结合有关,其浓度比抑制GM-CSF释放高10-100倍。24小时后,未观察到NF-kappaB、IkappaBalpha或I-kappaBbeta内源性抑制剂的诱导,糖皮质激素未改变IL-1β降解并随后诱导IkappaB的能力。4.丙酸氟替卡松(IC50=0.5 x 10(-11)M)、布地奈德(IC50=2.7 x 10(-11M))、地塞米松(IC50=0.5 x 10(-9)M)和RU486(IC50=2.7 x 10-11)抑制3 xκB的能力与抑制GM-CSF释放有关。5.这些数据表明,一系列糖皮质激素的抗炎特性与其反式加压而非反式激活基因的能力有关[2]。
|
| 细胞实验 |
糖皮质激素是一种广泛应用于临床实践的抗炎药。越来越多的证据表明外泌体是炎症的重要介质,但糖皮质激素是否调节外泌体的分泌和功能尚不清楚。在本研究中,我们观察到经地塞米松处理后,脂多糖(LPS)诱导的RAW264.7巨噬细胞的外泌体分泌减少。重要的是,从LPS诱导的RAW264.7巨噬细胞中分离的外泌体增加了RAW264.6细胞中TNF-α和IL-6的产生。然而,在用从地塞米松处理的细胞中分离的外泌体治疗后,这种增加不太明显。此外,地塞米松降低了LPS诱导的RAW264.7巨噬细胞外泌体中促炎微小RNA-155的表达。我们假设外泌体是糖皮质激素在LPS诱导的巨噬细胞炎症反应中抗炎作用的新靶点。这些发现将有利于抗炎治疗新方法的开发[5]。
|
| 动物实验 |
We investigated the role that mitochondrial proton leak may play in the glucocorticoid-induced hypermetabolic state. Sprague-Dawley rats were injected with dexamethasone over a period of 5 days. Liver mitochondria and gastrocnemius subsarcolemmal and intermyofibrillar mitochondria were isolated from dexamethasone-treated, pair-fed and control rats. Respiration and membrane potential were measured simultaneously using electrodes sensitive to oxygen and to the potential-dependent probe triphenylmethylphosphonium, respectively. Five days of dexamethasone injection resulted in a marked increase in the basal proton conductance of liver mitochondria, but not in the muscle mitochondrial populations. This effect would have a modest impact on energy expenditure in rats.[4]
|
| 参考文献 |
[1]. LaLone CA, et al. Effects of a glucocorticoid receptor agonist, Dexamethasone, on fathead minnow reproduction, growth, and development. Environ Toxicol Chem. 2012 Mar;31(3):611-22.
[2]. Adcock IM, et al. Ligand-induced differentiation of glucocorticoid receptor (GR) trans-repression and transactivation: preferential targetting of NF-kappaB and lack of I-kappaB involvement. Br J Pharmacol. 1999 Jun;127(4):1003-11. [3]. Rocksén D, et al. Differential anti-inflammatory and anti-oxidative effects of Dexamethasone and N-acetylcysteine in endotoxin-induced lung inflammation. Clin Exp Immunol. 2000 Nov;122(2):249-56. [4]. Roussel D, et al. Dexamethasone treatment specifically increases the basal proton conductance of rat liver mitochondria. FEBS Lett. 2003 Apr 24;541(1-3):75-9. [5]. Yun Chen, et al. Glucocorticoids inhibit production of exosomes containing inflammatory microRNA-155 in lipopolysaccharide-induced macrophage inflammatory responses. Int J Clin Exp Pathol 2018;11(7):3391-3397. |
| 其他信息 |
Dexamethasone sodium phosphate is an organic sodium salt which is the disodium salt of dexamethasone phosphate. It has a role as a glucocorticoid receptor agonist. It contains a dexamethasone phosphate(2-).
Dexamethasone Sodium Phosphate is a sodium phosphate salt form of Dexamethasone, a synthetic adrenal corticosteroid with potent anti-inflammatory properties. In addition to binding to specific nuclear steroid receptors, dexamethasone also interferes with NF-kB activation and apoptotic pathways. This agent lacks the salt-retaining properties of other related adrenal hormones. (NCI04) See also: Dexamethasone (broader); Dexamethasone sodium phosphate; neomycin sulfate (component of) ... View More ... |
| 精确质量 |
516.13
|
|---|---|
| 元素分析 |
C, 51.17; H, 5.47; F, 3.68; Na, 8.90; O, 24.78; P, 6.00
|
| CAS号 |
2392-39-4
|
| 相关CAS号 |
Dexamethasone;50-02-2;Dexamethasone acetate;1177-87-3;Dexamethasone phosphate;312-93-6
|
| PubChem CID |
16961
|
| 外观&性状 |
Typically exists as white to off-white solids at room temperature
|
| 密度 |
1.32g/cm3
|
| 沸点 |
669.6ºC at 760 mmHg
|
| 熔点 |
233-235 °C
|
| 闪点 |
358.7ºC
|
| 蒸汽压 |
2.81E-15mmHg at 25°C
|
| 折射率 |
1.591
|
| LogP |
2.889
|
| tPSA |
156.83
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
34
|
| 分子复杂度/Complexity |
962
|
| 定义原子立体中心数目 |
8
|
| SMILES |
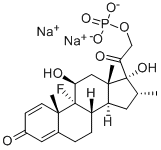
C[C@@H]1C[C@H]2[C@@H]3CCC4=CC(=O)C=C[C@@]4([C@]3([C@H](C[C@@]2([C@]1(C(=O)COP(=O)([O-])[O-])O)C)O)F)C.[Na+].[Na+]
|
| InChi Key |
PLCQGRYPOISRTQ-FCJDYXGNSA-L
|
| InChi Code |
InChI=1S/C22H30FO8P.2Na/c1-12-8-16-15-5-4-13-9-14(24)6-7-19(13,2)21(15,23)17(25)10-20(16,3)22(12,27)18(26)11-31-32(28,29)30/h6-7,9,12,15-17,25,27H,4-5,8,10-11H2,1-3H3,(H2,28,29,30)/q2*+1/p-2/t12-,15+,16+,17+,19+,20+,21+,22+/m1../s1
|
| 化学名 |
sodium 2-((8S,9R,10S,11S,13S,14S,16R,17R)-9-fluoro-11,17-dihydroxy-10,13,16-trimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl)-2-oxoethyl phosphate
|
| 别名 |
Dexamethasone 21-phosphate disodium EGP-437 EGP 437 EGP437 Dex-Phos.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ≥ 100 mg/mL (~193.65 mM)
DMSO : ~1 mg/mL (~1.94 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 100 mg/mL (193.65 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02955641 | UNKNOWN STATUSV | Drug: Dexamethasone Disodium Phosphate 0.1% Drug: Nepafenac 0.1% Drug: Hydroxyethylcellulose 0.19% |
Glaucoma, Angle-Closure Glaucoma, Closed-Angle Glaucoma, Narrow-Angle |
Sheba Medical Center | 2016-11-01 | Not Applicable |
| NCT01004497 | COMPLETED | Drug: Dasatinib Drug: Cyclophosphamide Drug: Vincristine |
Acute Lymphoblastic Leukemia | The Catholic University of Korea | 2010-03 | Phase 2 |
| NCT06437054 | NOT YET RECRUITING | Drug: Dexamethasone Drug: Hyaluronic acid Other: Indocyanine green(ICG) |
Hearing Loss, Sensorineural Hearing Loss, Sudden Intratympanic Injection |
Seoul National University Hospital | 2025-02-15 | Phase 1 Phase 2 |
| NCT03580473 | COMPLETED | Drug: SATURNO II association Drug: Vigadexa® |
Cataract Ocular Inflammation |
EMS | 2020-02-27 | Phase 2 |
| NCT02973880 | COMPLETED | Drug: NETILDEX™ ophthalmic gel Drug: NETILDEX™ eye drops solution |
Cataract Cataract Extraction |
SIFI SpA | 2017-10-15 | Phase 3 |
|
|
|