| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
Quinolone
|
|---|---|
| 体内研究 (In Vivo) |
表达编码海肾荧光素酶的 HCV 复制子的稳定细胞系(Huh-7-Lunet 或 Huh7-1C 细胞)。在基因型 1 至 6 中,vitilaprevir 表现出强大的全基因型抗病毒活性,EC50 范围为 0.33 至 6.6 nM。 Voxilaprevir 对 HCV 复制子株 DQ314805、H77、Con1、JFH-1、J6、J8(全长)的 IC50 值为 0.33 nM、3.9 nM、3.3 nM、3.7 nM、4.5 nM、1.8 nM 和 6.6 nM、1.9 nM 、HM568433、SA13(NS3嵌合体),分别[1]。
|
| 动物实验 |
Animal Model: Mice with a neutropenic murine lung infection model (four S. aureus , four S. pneumoniae, and four K. pneumoniae strains)[1]
Dosage: The total daily doses vary from 0.156 to 640 mg/kg/24 h Administration: 0.03 to 160 mg/kg are administered every 6 h (q6h) to infected mice by subcutaneous injection Result: Inhibited S. aureus strains ATCC 29213, ATCC 33591, MW2, R2527 with MICs of 0.008, 0.008, 0.004, and 0.004 mg/L, respectively. Inhibited S. pneumoniae strains ATCC 10813, ATCC 49619, 145, and 1329 with MICs of 0.03, 0.125, 0.016, and 0.016 mg/L, respectively. Inhibited K. pneumonia strains ATCC 43816, 4105, 4110, and 81-1260A with MICs of 0.06, 1, 0.5, and 0.06 mg/L, respectively. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The median time to peak plasma concentration for orally administered Delafloxacin is 0.75 (0.5-4.0) hours after a single dose and 1.00 (0.5-6.0) hours for steady state dosing. The median time to peak plasma concentration for intravenously administered Delafloxacin is 1.00 (1.0-1.2) hours for a single dose and 1.0 (1.0-1.0) hour for steady state dosing. The absolute bioavailability for orally administed Delafloxacin is 58.8%. After a single intravenous dose, 65% of Delafloxacin was excreted in the urine either unchanged or as glucuronide metabolites with 28% excreted unchanged in the feces. After a single oral dose, 50% of Delafloxacin was excreted in the urine either unchanged or as glucuronide metabolites with 48% excreted unchanged in the feces. The steady sate volume of distrubution of Delafloxacin is 30-48 liters. The mean total clearance of Delafloxacin is 16.3 liters per hour. Renal clearance accounts for 35-45% of total clearance. Metabolism / Metabolites Delafoxacin is primarily metabolized via glucuronidation mediated by UDP glucuronosyltransferase 1-1, UDP-glucuronosyltransferase 1-3, and UDP-glucuronosyltransferase 2B15. Less than 1% is metabolized via oxidation. Biological Half-Life The mean half life of elimination of Delafloxacin is 3.7 hours after a single intravenous administration. The mean half life of elimination for multple oral administrations is 4.2-8.5 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Delafloxacin, like other fluoroquinolones, is associated with a low rate (3% to 4%) of serum enzyme elevations during therapy. These abnormalities are generally mild, asymptomatic and transient, resolving even with continuation of therapy. ALT elevations above 5 times the upper limit of normal occur in 1% or less of subjects. Although delafloxacin may not have been clearly linked to cases of clinically apparent liver injury, the other fluoroquinolones, such as ciprofloxacin, levofloxacin and moxifloxacin, rank among the 25 most common causes of drug induced liver injury in many case series. Estimates of the frequency of liver injury from fluoroquinolones have been 1:15,000 to 1:25,000 exposed persons. Delafloxacin has been in clinical use for a short time only, but is likely to have a similar frequency and pattern of liver injury as the other fluoroquinolones. The typical presentation of fluoroquinolone associated liver injury is with a short latency (1 day to 3 weeks) and abrupt onset with nausea, fatigue, abdominal pain and jaundice. The pattern of serum enzyme elevations can be either hepatocellular or cholestatic, cases with the shorter times to onset usually being more hepatocellular. In addition, the onset of illness may occur a few days after the medication is stopped. Many (but not all) cases have prominent allergic manifestations with fever and rash, and the liver injury may occur in the context of a generalized hypersensitivity reaction. Autoantibodies are usually not present. Most reported cases of liver injury from fluoroquinolones have been mild and self-limited, with recovery in 4 to 8 weeks from onset. However, the fatality rate of cases with jaundice has been greater than 10%. In addition, cases with a cholestatic pattern of serum enzymes may run a prolonged course and, in rare instances, have progressed to chronic vanishing bile duct syndrome leading to liver failure. Nevertheless, delafloxacin is a relatively recently introduced antibiotic and has yet to be convincingly linked to instances of acute hepatitis or jaundice. Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of delafloxacin during breastfeeding. Fluoroquinolones have traditionally not been used in infants because of concern about adverse effects on the infants' developing joints. However, recent studies indicate little risk. The calcium in milk might prevent absorption of fluoroquinolones in milk, but insufficient data exist to prove or disprove this assertion. Use of delafloxacin is acceptable in nursing mothers. However, it is preferable to use an alternate drug for which safety information is available. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Delafloxacin is 84% bound to human plasma proteins. It primarily binds to serum albumin. |
| 参考文献 | |
| 其他信息 |
Delafloxacin is a fluoroquinolone antibiotic which has been used in trials studying the treatment and basic science of Gonorrhea, Hepatic Impairment, Bacterial Skin Diseases, Skin Structure Infections, and Community Acquired Pneumonia, among others. It was approved in June 2017 under the trade name Baxdela for use in the treatment of acute bacterial skin and skin structure infections.
Delafloxacin is a Fluoroquinolone Antibacterial. Delafloxacin is a fourth generation fluoroquinolone with expanded activity against gram-positive bacteria as well as atypical pathogens. Delafloxacin has been linked to mild ALT elevations during therapy, but has yet to be linked to instances of idiosyncratic acute liver injury with symptoms and jaundice as have been described with other fluoroquinolones. See also: Delafloxacin Meglumine (active moiety of). Drug Indication Delafloxacin is indicated for the treatment of acute bacterial skin and skin structure infections caused by the Gram-positive organisms Staphylococcus aureus (including methicillin-resistant and methicillin-susceptible isolates), Staphylococcus haemolyticus, Staphylococcus lugdunensis, Streptococcus agalactiae, Streptococcus anginosus Group (including Streptococcus anginosus, Streptococcus intermedius, and Streptococcus constellatus), Streptococcus pyogenes, and Enterococcus faecalis as well as the Gram-negative organisms Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae, and Pseudomonas aeruginosa. FDA Label Quofenix is indicated for the treatment of the following infections in adults: acute bacterial skin and skin structure infections (ABSSSI),community-acquired pneumonia (CAP), when it is considered inappropriate to use other antibacterial agents that are commonly recommended for the initial treatment of these infections (see sections 4. 4 and 5. 1). Consideration should be given to official guidance on the appropriate use of antibacterial agents. Treatment of community-acquired pneumonia Treatment of local infections of skin and subcutaneous tissues Treatment of local infections of skin and subcutaneous tissues Mechanism of Action Delafloxacin inhibits the activity of bacterial DNA topoisomerase IV and DNA gyrase (topoisomerase II). This interferes with bacterial DNA replication by preventing the relaxation of positive supercoils introduced as part of the elongation process. The resultant strain inhibits further elongation. Delafloxacin exerts concentration-dependent bacteriocidal activity. |
| 分子式 |
C18H12CLF3N4O4
|
|---|---|
| 分子量 |
440.76
|
| 精确质量 |
440.049
|
| 元素分析 |
C, 49.05; H, 2.74; Cl, 8.04; F, 12.93; N, 12.71; O, 14.52
|
| CAS号 |
189279-58-1
|
| 相关CAS号 |
Delafloxacin meglumine;352458-37-8;Delafloxacin-d5
|
| PubChem CID |
487101
|
| 外观&性状 |
White to off-white solid powder.
|
| 密度 |
1.8±0.1 g/cm3
|
| 沸点 |
698.5±55.0 °C at 760 mmHg
|
| 闪点 |
376.2±31.5 °C
|
| 蒸汽压 |
0.0±2.3 mmHg at 25°C
|
| 折射率 |
1.717
|
| LogP |
0.81
|
| tPSA |
122.41
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
30
|
| 分子复杂度/Complexity |
755
|
| 定义原子立体中心数目 |
0
|
| SMILES |
ClC1=C2C(C(C(C(=O)O[H])=C([H])N2C2=C(C([H])=C(C(N([H])[H])=N2)F)F)=O)=C([H])C(=C1N1C([H])([H])C([H])(C1([H])[H])O[H])F
|
| InChi Key |
DYDCPNMLZGFQTM-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C18H12ClF3N4O4/c19-12-13-7(1-9(20)14(12)25-3-6(27)4-25)15(28)8(18(29)30)5-26(13)17-11(22)2-10(21)16(23)24-17/h1-2,5-6,27H,3-4H2,(H2,23,24)(H,29,30)
|
| 化学名 |
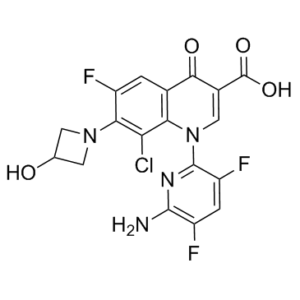
1-(6-Amino-3,5-difluoropyridin-2-yl)-8-chloro-6-fluoro-7-(3-hydroxyazetidin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid
|
| 别名 |
Trade name. Baxdela; ABT-492; RX 3341; WQ-3034; ABT 492; RX3341; WQ3034; ABT492; RX-3341; WQ 3034
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 88~100 mg/mL ( 199.65~226.88 mM )
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2688 mL | 11.3440 mL | 22.6881 mL | |
| 5 mM | 0.4538 mL | 2.2688 mL | 4.5376 mL | |
| 10 mM | 0.2269 mL | 1.1344 mL | 2.2688 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Time-kill curves for four clinical isolates. Curves on the left represent the activity of ABT-492, and the curves on the right represent the activity of levofloxacin against the same isolate.Antimicrob Agents Chemother.2004 Jan;48(1):203-8. |
|---|
Composite concentration-response curves for penicillin-sensitiveS. pneumoniae(SPS) and penicillin-resistantS. pneumoniae(SPR) isolates following antibiotic exposure to ABT-492 (left-hand side) and levofloxacin (right-hand side) at 4, 6, and 12 h.Antimicrob Agents Chemother.2004 Jan;48(1):203-8. |