| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
S1PR2 (sphingosine 1-phosphate receptor 2)
|
|---|---|
| 体外研究 (In Vitro) |
CYM-5520 是野生型 S1PR2 的完全激动剂,EC50 为 1.6 μM。虽然 CYM-5520 是一种 EC50 为 1.5 μM 的激动剂,但用 S1P 刺激表达三重突变体 S1PR2 的细胞不会刺激荧光素酶活性 [1]。
|
| 体内研究 (In Vivo) |
骨质减少切除手术后,CYM-5520(10 mg/kg;腹腔注射;每周 5 天;持续 6 周)治疗显着增加长骨和椎骨骨量。此外,CYM-5520 还可提高碱性磷酸酶、成骨细胞计数和前胶原 IC 末端前肽(骨合成代谢标志物)的浓缩浓度 [2]。
|
| 酶活实验 |
33P-S1P放射性配体竞争结合试验[1]
采用Sphingosine, D-erythro [33P] 1-phosphate。将S1PR2-CRE bla细胞接种到24孔板的孔中,20万个细胞加入1.0 mL生长培养基,在100%湿度、5% co2、37℃的培养箱中培养过夜。检测前将培养基替换为1% CDS血清培养基4小时。在4°C时,将培养基去除,并在结合缓冲液(20 mM Tris, pH 7.5, 100 mM NaCl, 15 mM NaF和新鲜添加的1 mM Na3VO4和蛋白酶抑制剂)中替换为测试化合物或载体对照。 Glo-sensor cAMP瞬时转染试验[1] 使用Fugene HD将GloSensor载体(pGLoSensor-20FcAMP, Promega)转染到S1PR2-eGFP或S1PR2-TM-eGFP Jump-In CHO细胞中。第二天,用0.05%胰蛋白酶EDTA收获细胞,在含有2%糖葡聚糖剥离血清的CO2独立培养基中重悬至500,000个细胞/mL,加入20 μL细胞悬液于384孔组织培养处理过的白板中,在37℃,5% CO2条件下孵育过夜。然后加入25 uL含有2%CDS和4% GloSensor Reagent的CO2独立培养基,室温孵育2小时。加入拮抗剂(JTE-013)或对照物孵育20分钟,然后加入激动剂化合物或S1P。15分钟后,在Perkin Elmer Envision平板阅读器上读取发光。 |
| 细胞实验 |
Jump-In CHO S1PR2野生型和头组三突变细胞系[1]
利用多位点Gateway克隆技术,从pEnter-15- S1pr2和pENTER-52-eGFP中获得帧内S1pr2- egfp表达构建体。将S1pr2-eGFP融合蛋白表达载体克隆到pDEST-CMV-JTI中。通过与S1PR1比对,确定了S1PR2头基团结合侧链。这些突变是通过重叠寡核苷酸PCR产生的。14通过重叠寡核苷酸PCR诱变产生三突变体S1PR2 (R108A、E109A和K269A)。所有构建体均经DNA测序证实。将这些载体转染到CHO JumpIn细胞中,并用10微克/毫升囊胚杀虫素进行筛选通过流式细胞仪对GFP阳性细胞进行分选,形成了一个均匀的细胞池。 |
| 动物实验 |
Animal/Disease Models: Ovariectomized 12weeks old C57Bl6J mice[2]
Doses: 10 mg/kg Route of Administration: ip; 5 days per week; 6 weeks Experimental Results: Correction of ovariectomized mice by inducing new bone formation Osteopenia. Ovariectomized 12 weeks old C57Bl6J mice were purchased from Charles River Laboratories. Mice were housed in SPF cages without any pathogens and with access to mouse chow and water ad libitum. Treatment was started 5 weeks after OVX. 1E)-1-(4-((1R,2S,3R)-1,2,3,4-Tetrahydroxybutyl)-1H-imidazol-2-yl)ethanone oxime (LX2931) was synthesized according to the known procedure and administered with the drinking water at 200 mg/kg/day for 6 weeks. CYM5520 was administered intraperitoneally at 10 mg/kg/day for 5 consecutive days per week for 6 weeks. The total number of mice used in the whole study was 21. Every effort was taken to minimize the number of animals used and their suffering.[2] |
| 参考文献 | |
| 其他信息 |
Molecular probe tool compounds for the Sphingosine 1-phosphate receptor 2 (S1PR2) are important for investigating the multiple biological processes in which the S1PR2 receptor has been implicated. Amongst these are NF-κB-mediated tumor cell survival and fibroblast chemotaxis to fibronectin. Here we report our efforts to identify selective chemical probes for S1PR2 and their characterization. We employed high throughput screening to identify two compounds which activate the S1PR2 receptor. SAR optimization led to compounds with high nanomolar potency. These compounds, XAX-162 and CYM-5520, are highly selective and do not activate other S1P receptors. Binding of CYM-5520 is not competitive with the antagonist JTE-013. Mutation of receptor residues responsible for binding to the zwitterionic headgroup of sphingosine 1-phosphate (S1P) abolishes S1P activation of the receptor, but not activation by CYM-5520. Competitive binding experiments with radiolabeled S1P demonstrate that CYM-5520 is an allosteric agonist and does not displace the native ligand. Computational modeling suggests that CYM-5520 binds lower in the orthosteric binding pocket, and that co-binding with S1P is energetically well tolerated. In summary, we have identified an allosteric S1PR2 selective agonist compound. [1]
Molecular probe tool compounds for the Sphingosine 1-phosphate receptor 2 (S1PR2) are important for investigating the multiple biological processes in which the S1PR2 receptor has been implicated. Amongst these are NF-κB-mediated tumor cell survival and fibroblast chemotaxis to fibronectin. Here we report our efforts to identify selective chemical probes for S1PR2 and their characterization. We employed high throughput screening to identify two compounds which activate the S1PR2 receptor. SAR optimization led to compounds with high nanomolar potency. These compounds, XAX-162 and CYM-5520, are highly selective and do not activate other S1P receptors. Binding of CYM-5520 is not competitive with the antagonist JTE-013. Mutation of receptor residues responsible for binding to the zwitterionic headgroup of sphingosine 1-phosphate (S1P) abolishes S1P activation of the receptor, but not activation by CYM-5520. Competitive binding experiments with radiolabeled S1P demonstrate that CYM-5520 is an allosteric agonist and does not displace the native ligand. Computational modeling suggests that CYM-5520 binds lower in the orthosteric binding pocket, and that co-binding with S1P is energetically well tolerated. In summary, we have identified an allosteric S1PR2 selective agonist compound.[2] |
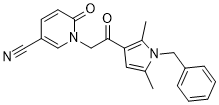
| 分子式 |
C21H19N3O2
|
|---|---|
| 分子量 |
345.394464731216
|
| 精确质量 |
345.147
|
| 元素分析 |
C, 73.03; H, 5.54; N, 12.17; O, 9.26
|
| CAS号 |
1449747-00-5
|
| PubChem CID |
25110470
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
580.5±50.0 °C at 760 mmHg
|
| 闪点 |
304.9±30.1 °C
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
| 折射率 |
1.611
|
| LogP |
2.52
|
| tPSA |
66.1
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
26
|
| 分子复杂度/Complexity |
666
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
FMYGNANMYYHBSU-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C21H19N3O2/c1-15-10-19(16(2)24(15)13-17-6-4-3-5-7-17)20(25)14-23-12-18(11-22)8-9-21(23)26/h3-10,12H,13-14H2,1-2H3
|
| 化学名 |
1-[2-[2,5-Dimethyl-1-(phenylmethyl)-1H-pyrrol-3-yl]-2-oxoethyl]-1,6-dihydro-6-oxo-3-pyridinecarbonitrile
|
| 别名 |
CYM-5520; CYM5520; 1449747-00-5; 1-[2-(1-Benzyl-2,5-dimethyl-1H-pyrrol-3-yl)-2-oxo-ethyl]-6-oxo-1,6-dihydro-pyridine-3-carbonitrile; 1-[2-[2,5-Dimethyl-1-(phenylmethyl)-1H-pyrrol-3-yl]-2-oxoethyl]-1,6-dihydro-6-oxo-3-pyridinecarbonitrile; 1-(2-(1-Benzyl-2,5-dimethyl-1H-pyrrol-3-yl)-2-oxoethyl)-6-oxo-1,6-dihydropyridine-3-carbonitrile; 1-[2-(1-BENZYL-2,5-DIMETHYLPYRROL-3-YL)-2-OXOETHYL]-6-OXOPYRIDINE-3-CARBONITRILE; CYM 5520.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~50 mg/mL (~144.76 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.24 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8953 mL | 14.4764 mL | 28.9528 mL | |
| 5 mM | 0.5791 mL | 2.8953 mL | 5.7906 mL | |
| 10 mM | 0.2895 mL | 1.4476 mL | 2.8953 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。