| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
游离 3'-OH DNA 末端的 TUNEL 标记表明,环磷酰胺会促进外膜起泡,导致 DNA 片段化,并促进 9L/P450 细胞中 caspase 3 和 caspase 7 底物 PARP 的裂解。在用活化环磷酰胺处理的细胞中,Bcl-2 表达完全阻止启动子和效应子 caspase 3 的激活。 Bcl-2 抑制细胞毒性作用,但不抑制活化环磷酰胺的细胞抑制作用 [1]。环磷酰胺可逆地抑制 AChE,IC50 为 511 μM [2]。四氯化碳不影响环磷酰胺或4-羟基环磷酰胺对培养细胞的直接细胞毒性[3]。
|
|---|---|
| 体内研究 (In Vivo) |
当腹膜内 (ip) 给予 SW1 肿瘤 C3H 小鼠(0.1 mL PBS 中 2 mg/只)时,环磷酰胺会增加脾脏和肿瘤中 CD3、CD4 或 CD8 染色的细胞百分比 [4]。
|
| 动物实验 |
Animal/Disease Models: Six- to eightweeks old female C3H/HeN mice bearing SW1 tumors [4]
Doses: 2 mg/mouse Route of Administration: intraperitoneal (ip) injection; 2 mg/mouse in 0.1 mL PBS; Four-day Experimental Results: spleen and an increase in the percentage of cells staining for CD3, CD4, or CD8 in the tumor. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Cyclophosphamide appears in milk in potentially toxic amounts; additionally, highly toxic active metabolites could add to the risk to the infant. Neutropenia has been reported in 2 infants whose mothers breastfed them while receiving cyclophosphamide. Most sources consider breastfeeding to be contraindicated during maternal cytotoxic antineoplastic drug therapy, especially alkylating agents such as cyclophosphamide. Although some have suggested withholding breastfeeding for 1 to 3 days after a dose, it appears to take more than 21 days for the drug and its metabolites to be completely eliminated from breastmilk. Some authors’ data suggest that it might take 6 weeks for milk levels to drop to a safe level after a dose of cyclophosphamide 750 mg/sq. m. Chemotherapy may adversely affect the normal microbiome and chemical makeup of breastmilk. Women who receive chemotherapy during pregnancy are more likely to have difficulty nursing their infant. ◉ Effects in Breastfed Infants In one 23-day-old infant, neutropenia, thrombocytopenia and a low hemoglobin were possibly caused by cyclophosphamide after 3 days of maternal treatment with cyclophosphamide 6 mg/kg IV daily (total dose 300 mg). In a 4-month-old, neutropenia was probably caused by cyclophosphamide in a mother 9 days after the last of 6 weekly doses of 800 mg cyclophosphamide intravenously, 2 mg vincristine intravenously and daily doses of 30 mg of prednisolone orally. Neutropenia persisted at least 12 days and was accompanied by a brief episode of diarrhea. A woman was diagnosed with B-cell lymphoma at 27 weeks of pregnancy. Labor was induced at 34 4/7 weeks and treatment was begun with a standard regimen of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone in unspecified doses on a 21-day cycle, starting on day 2 postpartum. She pumped and discarded her milk and fed her infant donor milk for the first 10 days of each cycle and then breastfed her infant for the remaining 10 days before the next treatment cycle. The 10-day period of breastfeeding abstinence was determined by using about 3 half-lives of vincristine. After completion of 4 cycles of chemotherapy, her infant was reportedly healthy and developing without any complications. ◉ Effects on Lactation and Breastmilk A case of euprolactinemic galactorrhea was reported in a 55-year-old woman receiving cyclophosphamide for pemphigus vulgaris. One month after starting cyclophosphamide 50 mg daily she experienced fullness in both breasts and bilateral milky nipple discharge. No hormonal abnormalities were found. After cyclophosphamide was discontinued, her symptoms resolved completely and did not recur. Galactorrhea was probably caused by cyclophosphamide. Telephone follow-up study was conducted on 74 women who received cancer chemotherapy at one center during the second or third trimester of pregnancy to determine if they were successful at breastfeeding postpartum. Only 34% of the women were able to exclusively breastfeed their infants, and 66% of the women reported experiencing breastfeeding difficulties. This was in comparison to a 91% breastfeeding success rate in 22 other mothers diagnosed during pregnancy, but not treated with chemotherapy. Other statistically significant correlations included: 1. mothers with breastfeeding difficulties had an average of 5.5 cycles of chemotherapy compared with 3.8 cycles among mothers who had no difficulties; and 2. mothers with breastfeeding difficulties received their first cycle of chemotherapy on average 3.4 weeks earlier in pregnancy. Of the 56 women who received a cyclophosphamide-containing regimen, 34 had breastfeeding difficulties. |
| 参考文献 |
|
| 其他信息 |
Cyclophosphamide monohydrate appears as fine white crystalline powder with a slightly bitter taste. Little or no odor. (NTP, 1992)
Cyclophosphamide hydrate is the monohydrate of cyclophosphamide. It has a role as an antineoplastic agent, an immunosuppressive agent, an alkylating agent and a carcinogenic agent. It contains a cyclophosphamide. Cyclophosphamide is a synthetic alkylating agent chemically related to the nitrogen mustards with antineoplastic and immunosuppressive activities. In the liver, cyclophosphamide is converted to the active metabolites aldophosphamide and phosphoramide mustard, which bind to DNA, thereby inhibiting DNA replication and initiating cell death. Precursor of an alkylating nitrogen mustard antineoplastic and immunosuppressive agent that must be activated in the LIVER to form the active aldophosphamide. It has been used in the treatment of LYMPHOMA and LEUKEMIA. Its side effect, ALOPECIA, has been used for defleecing sheep. Cyclophosphamide may also cause sterility, birth defects, mutations, and cancer. See also: Cyclophosphamide (annotation moved to). |
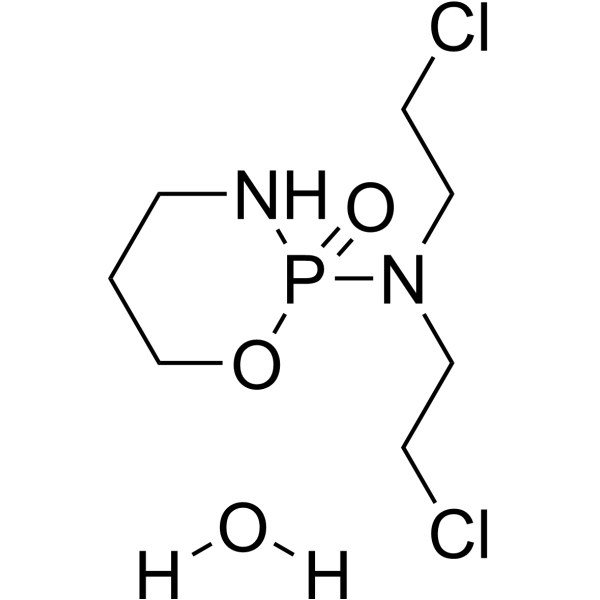
| 分子式 |
C7H17CL2N2O3P
|
|---|---|
| 分子量 |
279.1012
|
| 精确质量 |
278.035
|
| CAS号 |
6055-19-2
|
| 相关CAS号 |
Cyclophosphamide;50-18-0;Cyclophosphamide-d8 hydrate
|
| PubChem CID |
22420
|
| 外观&性状 |
White to off-white solid powder
|
| 沸点 |
336.1ºC at 760 mmHg
|
| 熔点 |
49-51 °C(lit.)
|
| 闪点 |
>230 °F
|
| LogP |
2.148
|
| tPSA |
60.61
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
15
|
| 分子复杂度/Complexity |
212
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
PWOQRKCAHTVFLB-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C7H15Cl2N2O2P.H2O/c8-2-5-11(6-3-9)14(12)10-4-1-7-13-14;/h1-7H2,(H,10,12);1H2
|
| 化学名 |
N,N-bis(2-chloroethyl)-2-oxo-1,3,2λ5-oxazaphosphinan-2-amine;hydrate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 该产品在溶液状态不稳定,请现配现用。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ≥ 50 mg/mL (~179.15 mM)
DMSO : ≥ 38 mg/mL (~136.15 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.96 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (8.96 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (8.96 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 25 mg/mL (89.57 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.5829 mL | 17.9147 mL | 35.8295 mL | |
| 5 mM | 0.7166 mL | 3.5829 mL | 7.1659 mL | |
| 10 mM | 0.3583 mL | 1.7915 mL | 3.5829 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。