| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other size |
|
| 体外研究 (In Vitro) |
黄连碱的 IC50 值为 6.3 μM,Ki 值为 5.8 μM,是一种高效的非竞争性 IDO 抑制剂[1]。共氧辛 (0.1-100 μM) 抑制 A549、H460、H2170、MDA-MB-231 和 HT-29 细胞的生长,IC50 值依次为 18.09、29.50、21.60、20.15 和 26.60 μM 。在 A549 细胞中,黄连碱(12.5、25 和 50 μM)浓度依赖性地导致 G2/M 停滞和细胞凋亡,下调细胞周期蛋白 B1、cdc2 和 cdc25C 的表达,并增加 pH2AX 和 p21 的表达。在 A549 细胞中,复合复合物(12.5、25、50 μM)还会导致线粒体功能障碍并触发 caspase 活性。此外,黄连碱 (50 μM) 会以时间依赖性方式(0.5、1、2、4、12 和 24 小时)升高 ROS 水平 [3]。
|
|---|---|
| 体内研究 (In Vivo) |
小鼠对黄连碱的LD50值为880.18 mg/kg,其毒性随浓度增加而增加。 154mg/kg/天的剂量连续90天对SD大鼠没有引起毒性。除了不同程度地增加 HDL-c 含量并减缓 HFHC 饮食带来的体重增加之外,copoxin(23.35、46.7、70.05 mg/kg,口服)还以剂量依赖性方式增加仓鼠粪便胆固醇和 TBA 水平方式。它还降低了动物血清中的 TC、TG 和 LDL-c 水平。黄连碱(70.05 mg/kg,口服)可诱导参与胆固醇代谢的 SREBP-2、LDLR 和 CYP7A1 蛋白的表达,从而降低 HMGCR 蛋白表达水平 [2]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Corydalis saxicola Bunting (Yanhuanglian) is an important component in various prescriptions in traditional Chinese medicine. Yanhuanglian has been demonstrated to possess many pharmacological activities, including antibacterial, antiviral, and anticancer activities. The active fractions are dehydrocavidine, coptisine, dehydroapocavidine, and tetradehydroscoulerine. The purpose of the present study was to examine in vivo pharmacokinetics and tissue distribution in rats by using high-performance liquid chromatography (HPLC) coupled with tandem mass spectrometry. Systemic clearance of the four active alkaloids in plasma was over 93% of hepatic blood flow, indicating they may be quickly eliminated via hepatic clearance. Less than 10% drugs was excreted via urine following intravenous and oral administration, suggesting that these four alkaloids may undergo significant metabolism in the body or the drug may be excreted via other routes other than urine. There was significantly lower excretion of these four alkaloids following oral than intravenous administration, suggesting a significant first pass effect after oral administration. There appeared to be wide distribution of those four alkaloids in rats as demonstrated by the higher apparent volume of distribution. Our results have also demonstrated that the four alkaloids can be absorbed following oral administration although there were less than 15% of drugs absorbed into systemic circulation. In summary, the favorable oral bioavailability properties of those four active alkaloids in rats make Yanhuanglian extract worth further investigation for improving oral bioavailability. To study the absorption of coptisine chloride (COP) and berberrubine (BRB) as chemical constituents of some traditional Chinese medicines in human intestinal epithelial. By using Caco-2 (the human colonic adenocarcinoma cell lines) cell monolayers as an intestinal epithelial cell model, the permeability of COP and BRB were studied from apical side (AP side) to basolateral side (BL side) or from BL side to AP side. The two alkaloids were measured by reversed-phase high performance liquid chromatography (HPLC) coupled with UV detector. Transport parameters and apparent permeability coefficients (P(app)) were then calculated and compared with those of propranolol and atenolol. P(app) values were also compared with the reported values for model compounds (propranolol and atenolol). The P(app) values of COP, BRB were (1.103 +/- 0.162) x 10(-5), (1.309 +/- 0.102) x 10(-5) cm x s(-1 from AP side to BL side, and (0.300 +/- 0.041) x 10(-5) and (1.955 +/- 0.055) x 10(-5) cm x s(-1) from BL side to AP side, respectively. Their P(app) values were identical with those of propranolol [(2.23 +/- 0.10) x 10(-5 cm x s(-1)], which is a transcellular transport marker and as a control substance for high permeability. On the other hand, the efflux transport of BRB was higher 1.49 times more than its influx transport with 0.67 rate of P(app A-->B)/P(app B-->A). But P(app A-->B)/P(app B-->A value of COP was 3.67, which suggested that the efflux transport have not been involved in its absorbed mechanism in Caco-2 cells monolayers. COP and BRB can be absorbed across intestinal epithelial cells, and they are completely absorbed compounds. BRB may have been involved in efflux mechanism in Caco-2 cells monolayers model from the basolateral-to-apical direction. To determine the pharmacokinetics, distribution, and mutual transformation of the total alkaloids, jatrorrhizine, coptisine, berberine, and palmatine from Coptis chinensis in rats. After the total alkaloids and berberine were fed into rats, their contents in plasma, tissues and gastrointestinal tract were determined by reversed-phase HPLC. The peak times of berberine in blood were 2.0 hr (Cmax 3.7 mg x L(-1)) and 5.0 hr Cmax 2.8 mg x L(-1)), respectively. Berberine in rat blood can be transformed into jatrorrhizine. After the rats were fed with the total alkaloids by gavage, the content of berberine was decreased monotonously, while coptisine, palmatine, and jatrorrhizine contents were increased gradually in the stomach, it speculated that berberine may be transformed into jatrorrhizine in the stomach. Animal experiments showed that berberine and palmatine were mainly distributed in the lungs of animals, followed by the distribution in the liver, while jatrorrhizine and coptisine was mainly in the liver, then in the lungs. Berberine could transform into jatrorrhizine. The mechanism on the appearance of two maximum blood concentration of berberine in blood could be explained with the propulsion of the gastrointestinal tract partly. The absorption and transport mechanisms of berberine, palmatine, jateorhizine, and coptisine were studied using a Caco-2 cells uptake and transport model, with the addition of cyclosporin A and verapamil as P-glycoprotein (P-gp) inhibitors and MK-571 as a multidrug resistance-associated protein 2 (MRP(2)) inhibitor. In the uptake experiment, berberine, palmatine, jateorhizine, and coptisine were all taken into Caco-2 cells, and their uptakes were increased in the presence of cyclosporin A or verapamil. In the transport experiment, P(app) (AP-BL) was between 0.1 and 1.0 x 10(6) cm/sec for berberine, palmatine, jateorhizine, and coptisine and was lower than P(app) (BL-AB). ER values were all >2. Cyclosporin A and verapamil both increased P(app) (AP-BL) but decreased P(app) (BL-AB) for berberine, palmatine, jateorhizine, and coptisine; ER values were decreased by >50%. MK-571 had no influence on the transmembrane transport of berberine, palmatine, jateorhizine, and coptisine. At a concentration of 1-100 uM, berberine, palmatine, jateorhizine, and coptisine had no significant effects on the bidirection transport of Rho123. Berberine, palmatine, jateorhizine, and coptisine were all P-gp substrates; and at the range of 1-100 uM, berberine, palmatine, jateorhizine, and coptisine had no inhibitory effects on P-gp. Jiao-Tai-Wan (JTW), an important herbal formula consists of Rhizoma coptidis and Cortex cinnamomi powder, is a famous prescription which has been used for centuries to treat insomnia in Traditional Chinese Medicine. The purpose of this study is to compare the pharmacokinetic properties of five protoberberine-type alkaloids (i.e. berberine, palmatine, coptisine, epiberberine and jatrorrhizine), the main bioactive constituents in JTW, between normal and insomnic rats. We also investigate the differences between single-dose and multiple-dose pharmacokinetics of five protoberberine-type alkaloids. The insomnic rat models were induced by intraperitoneal injection of one-dose para-chlorophenylalanine acid (PCPA). Quantification of five protoberberine-type alkaloids in rat plasma was achieved by using a rapid LC-MS/MS method. Plasma samples were collected at different time points to construct pharmacokinetic profiles by plotting drug concentration versus time and estimate pharmacokinetic parameters. An unpaired Student's t test was used for comparisons with SPSS 17.0. The five protoberberine-type alkaloids of single-dose normal groups had slow absorption and low bioavailability, as well as a delay of peak time. In the single-dose oral administration, the Cmax and Tmax of five ingredients in insomnic rats had significant differences compared with those of normal rats. In the multiple-dose oral administration, the pharmacokinetic parameters of five protoberberine-type alkaloids varied greatly in insomnic rats. In the normal rats, there were significant differences (p<0.05) in the principal pharmacokinetic parameters such as Cmax and Tmax between single-dose and multiple-dose oral administration. In the insomnic rats, the five ingredients of multiple-dose groups showed better absorption than the single-dose groups. Particularly, three peaks were observed in multiple-dose model group of plasma-concentration curves. The pharmacokinetic behavior of five protoberberine-type alkaloids was described in this paper. In both normal groups and model groups, the pharmacokinetic behavior of multiple-dose had significant differences comparing with the single-dose; either single-dose or multiple-dose, the pharmacokinetic behavior of insomnic rats had significant differences comparing the normal rats. Multiple dosing may improve the absorption of JTW in insomnic rats, which will increase the bioavailability and bring into active role in therapeutical effect. |
| 参考文献 |
|
| 其他信息 |
Coptisine is an alkaloid. It has a role as a metabolite.
Coptisine has been reported in Coptis omeiensis, Corydalis solida, and other organisms with data available. See also: Sanguinaria canadensis root (part of); Chelidonium majus flowering top (part of). |
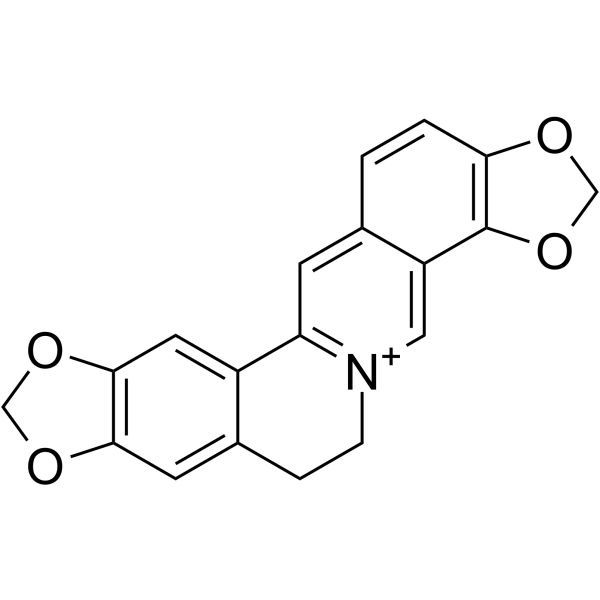
| 分子式 |
C19H14NO4
|
|---|---|
| 分子量 |
320.3188
|
| 精确质量 |
320.092
|
| CAS号 |
3486-66-6
|
| 相关CAS号 |
Coptisine Sulfate;1198398-71-8;Coptisine chloride;6020-18-4
|
| PubChem CID |
72322
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 熔点 |
212-217℃
|
| LogP |
-0.87
|
| tPSA |
40.8
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
0
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
502
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O1C([H])([H])OC2=C1C([H])=C1C(=C2[H])C([H])([H])C([H])([H])[N+]2C([H])=C3C4=C(C([H])=C([H])C3=C([H])C=21)OC([H])([H])O4
|
| InChi Key |
XYHOBCMEDLZUMP-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C19H14NO4/c1-2-16-19(24-10-21-16)14-8-20-4-3-12-6-17-18(23-9-22-17)7-13(12)15(20)5-11(1)14/h1-2,5-8H,3-4,9-10H2/q+1
|
| 化学名 |
5,7,17,19-tetraoxa-13-azoniahexacyclo[11.11.0.02,10.04,8.015,23.016,20]tetracosa-1(13),2,4(8),9,14,16(20),21,23-octaene
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1219 mL | 15.6094 mL | 31.2188 mL | |
| 5 mM | 0.6244 mL | 3.1219 mL | 6.2438 mL | |
| 10 mM | 0.3122 mL | 1.5609 mL | 3.1219 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。