| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|

| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Topically, chlorhexidine is unlikely to undergo any degree of systemic absorption. Orally administered chlorhexidine, such as that found in oral rinses for dental purposes, is very poorly absorbed from the gastrointestinal tract - the Cmax in human subjects following an oral dose of 300mg was 0.206 µg/g and occurred approximately 30 minutes after ingestion (Tmax). Following the insertion of 4 PerioChips in 18 adult patients, no detectable plasma or urine chlorhexidine levels were observed. Excretion of chlorhexidine gluconate occurs almost exclusively via the feces, with less than 1% of an ingested dose excreted in the urine. 34 newborn infants who had been bathed in a standard manner with Hibiscrub were studied to find out whether it was absorbed percutaneously. Low levels of chlorhexidine were found in the blood of all 10 babies sampled by heel prick, and 5 of 24 from whom venous blood was taken. /Chlorhexidine gluconate/ Percutaneous absorption of the antimicrobial agent chlorhexidine (labelled with carbon-14) was studied in rats. Less than 5% of the topically applied chlorhexidine was absorbed during a 5-day period. Excretion of absorbed radioactivity occurred mainly in the feces. The percutaneous absorption of chlorhexidine gluconate (chlorhexidine digluconate; Hibitane) through hairless rat skin with or without stratum corneum was studied. For tests carried out on whole skin, storage in cutaneous structures after 48 hr was more important than diffusion; the reverse was observed for stripped skin. When the skin was stripped, the amount absorbed was multiplied by approximately 100, and the amount stored in skin by approximately 10. The difference in chlorhexidine diffusion observed between whole and stripped skin was related to the physicochemical characteristics of chlorhexidine. /Chlorhexidine gluconate/ To evaluate the elimination kinetics of chlorhexidine in milk when used as an intramammary infusion to stop lactation in cows. ... The study was performed in 2 phases. Three cows were studied in each phase. All cows were treated with chlorhexidine suspension by infusion into a mastitic mammary gland quarter after 2 milkings 24 hours apart. Foremilk samples (100 mL) were collected from treated and untreated (controls) mammary gland quarters of each cow. Chlorhexidine was extracted from raw milk, and residue concentrations were quantified by use of high-performance liquid chromatography. Foremilk samples from days 2, 5, and 8 were analyzed in phase I, and samples from time 0 and days 3, 7, 14, 21, 28, 35, and 42 were analyzed in phase II. In phases I and II, there was no quantifiable transference of chlorhexidine to milk in untreated mammary gland quarters. Measurable chlorhexidine residues were found in milk from treated mammary gland quarters of 2 cows throughout the 42-day sample period in phase II. Estimated mean elimination half-life for chlorhexidine in milk was 11.5 days. Metabolism / Metabolites As chlorhexidine is very poorly absorbed in the gastrointestinal tract, it is unlikely to undergo metabolic conversion to any significant extent. Biological Half-Life To evaluate the elimination kinetics of chlorhexidine in milk when used as an intramammary infusion to stop lactation in cows. ... The study was performed in 2 phases. Three cows were studied in each phase. All cows were treated with chlorhexidine suspension by infusion into a mastitic mammary gland quarter after 2 milkings 24 hours apart. Foremilk samples (100 mL) were collected from treated and untreated (controls) mammary gland quarters of each cow. Chlorhexidine was extracted from raw milk, and residue concentrations were quantified by use of high-performance liquid chromatography. Foremilk samples from days 2, 5, and 8 were analyzed in phase I, and samples from time 0 and days 3, 7, 14, 21, 28, 35, and 42 were analyzed in phase II. In phases I and II, there was no quantifiable transference of chlorhexidine to milk in untreated mammary gland quarters. Measurable chlorhexidine residues were found in milk from treated mammary gland quarters of 2 cows throughout the 42-day sample period in phase II. Estimated mean elimination half-life for chlorhexidine in milk was 11.5 days. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Chlorhexidine forms solid crystals. Chlorhexidine diacetate is registered for current use in the U.S., but approved pesticide uses may change periodically and so federal, state and local authorities must be consulted for currently approved uses. Currently, two end-use products with 2% chlorhexidine diacetate are registered for use as hard surface-treatment disinfectant/virucides. Chlorhexidine is used primarily as its salts e.g., the dihydrochloride, diacetate, and digluconate in disinfectants (disinfection of the skin and hands), cosmetics (additive to creams, toothpaste, deodorants, and antiperspirants), and pharmaceutical products (preservative in eyedrops, active substance in wound dressings and antiseptic mouthwashes). HUMAN EXPOSURE AND TOXICITY: Chlorhexidine diacetate is highly acutely toxic when applied to the eye. Skin reactions to chlorhexidine-acetate and chlorhexidine-gluconate were tested among eczema patients. Positive reactions were found in 52 (5.4%) of the 1,063 subjects at the initial test. Of these subjects, 29 were retested, and 21 were still found to have positive reactions. Chlorhexidine specific IgE was detected only in Japanese individuals who had experienced anaphylactic type reactions and was not detected in Japanese nurses and patients or in a group of British nurses and hospital staff, all having regular contact with chlorhexidine. All chromogens plus chlorhexidine, but not chlorhexidine alone, produced some discoloration of hydroxyapatite and human teeth. A 67-yr-old man undergoing a colectomy for colon cancer was unintentionally administered 0.8 mg of chlorhexidine gluconate intravenously and subsequently developed acute respiratory distress syndrome. Occupational asthma has been described in two health care workers, as a result of exposure to chlorhexidine and alcohol aerosols. Another case report describes six patients who developed urticaria, dyspnea, and anaphylactic shock due to topical application of chlorhexidine gluconate solution. Even very dilute solutions of chlorhexidine can cause marked chondrolysis of articular cartilage leading to severe permanent damage to the knee. ANIMAL STUDIES: Rabbits suffered severe ocular irritation with chlorhexidine acetate treatment. No dermal irritation was reported up to 72 hours following test article treatment in rabbits. In developmental studies no observable malformations or developmental toxicity were found at any dose level tested. Both positive and negative results have been seen in bacterial studies of the mutagenic effects of chlorhexidine; however, no mutagenic activity was seen in an in-vivo micronucleus assay or a mammalian cytogenic test using Chinese-hamster-ovary cells. No carcinogenic effects were seen in a long term animal study. Interactions Chlorhexidine increases the activity of itraconazole against Candida isolates; itraconazole-chlorhexidine combinations show synergistic activity in culture media. Non-Human Toxicity Values LD50 Rat oral 5,000 mg/kg LD50 Rat (male) oral 1710 mg/kg /Chlorhexidine diacetate/ LD50 Rat (female) oral 1180 mg/kg /Chlorhexidine diacetate/ LD50 Rabbit dermal >2000 mg/kg /Chlorhexidine diacetate/ For more Non-Human Toxicity Values (Complete) data for CHLORHEXIDINE (6 total), please visit the HSDB record page. |
| 其他信息 |
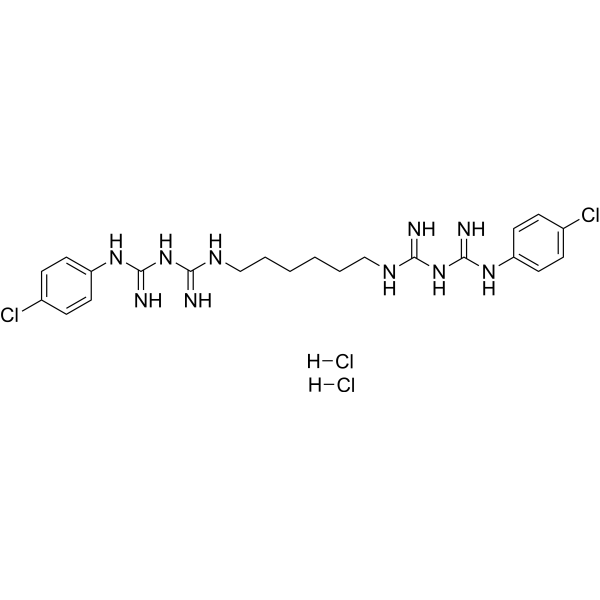
Chlorhexidine hydrochloride is a member of guanidines.
Chlorhexidine is a broad-spectrum antimicrobial biguanide used as a topical antiseptic and in dental practice for the treatment of inflammatory dental conditions caused by microorganisms. It is one of the most common skin and mucous membrane antiseptic agents in use today. The molecule itself is a cationic bis-guanide consisting of two 4-chlorophenyl rings and two biguanide groups joined by a central hexamethylene chain. Topical chlorhexidine for disinfection, as well as oral rinses for dental use, carries activity against a broad range of pathogens including bacteria, yeasts, and viruses. Chlorhexidine was developed in the UK by Imperial Chemical Industries in the early 1950s and was introduced to the US in the 1970s. The FDA withdrew its approval for the use of chlorhexidine gluconate topical tincture 0.5%, due to a significant number of reports concerning chemical and thermal burns associated with the use of this product. Other formulations of chlorhexidine continue to be available. Chlorhexidine is a biguanide compound used as an antiseptic agent with topical antibacterial activity. Chlorhexidine is positively charged and reacts with the negatively charged microbial cell surface, thereby destroying the integrity of the cell membrane. Subsequently, chlorhexidine penetrates into the cell and causes leakage of intracellular components leading to cell death. Since gram positive bacteria are more negatively charged, they are more sensitive to this agent. A disinfectant and topical anti-infective agent used also as mouthwash to prevent oral plaque. Drug Indication Chlorhexidine is available over-the-counter in various formulations (e.g. solution, sponge, cloth, swab) as a topical antiseptic to sanitize prior to surgeries and/or medical procedures. Dental formulations, available by prescription only, include an oral rinse indicated for the treatment of gingivitis and a slow-release "chip" which is inserted into periodontal pockets and is indicated for the reduction of pocket depth in adult patients with periodontitis as an adjunct therapy to dental scaling and root planing procedures. FDA Label Mechanism of Action Chlorhexidine’s broad-spectrum antimicrobial effects are due to its ability to disrupt microbial cell membranes. The positively charged chlorhexidine molecule reacts with negatively charged phosphate groups on microbial cell surfaces - this reaction both destroys the integrity of the cell, allowing leakage of intracellular material, and allows chlorhexidine to enter the cell, causing precipitation of cytoplasmic components and ultimately cell death. The specific means of cell death is dependent on the concentration of chlorhexidine - lower concentrations are bacteriostatic and result in leakage of intracellular substances such as potassium and phosphorous, whereas higher concentrations are bactericidal and cause cytoplasmic precipitation. Therapeutic Uses Antiseptic; disinfectant. (Vet): antiseptic; disinfectant. Cleanser: As a surgical hand scrub, skin wound and general skin cleanser, health care personnel hand wash, and for preoperative skin preparation. Chlorhedine gluconate significantly reduces the number of microorganisms on the hands and forearms prior to surgery or patient care. /Chlorhexidine gluconate-topical/ EXPL THER To determine if chlorhexidine can be used as an intervention to prolong the time to relapse of oral candidiasis. SUBJECTS AND METHODS: A double-blinded randomized clinical trial was performed in 75 HIV/AIDS subjects with oral candidiasis. Clotrimazole troche was prescribed, and the subjects were re-examined every 2 weeks until the lesions were completely eradicated. The subjects were then randomly divided into two groups; 0.12% chlorhexidine (n = 37, aged 22-52 years, mean 34 years) and 0.9% normal saline (n = 38, aged 22-55 years, mean 38 years). They were re-examined every 2 weeks until the next episode was observed. RESULTS: The time to recurrence of oral candidiasis between the chlorhexidine and the saline group was not statistically significant (P > 0.05). The following variables were significantly associated with the time of recurrence; frequency of antifungal therapy (P = 0.011), total lymphocyte (P = 0.017), alcohol consumption (P = 0.043), and candidiasis on gingiva (P = 0.048). The subjects with lower lymphocyte showed shorter oral candidiasis-free periods (P = 0.034). CONCLUSIONS: Chlorhexidine showed a small but not statistically significant effect in maintenance of oral candidiasis-free period. This lack of significance may be due to the small sample size. Further study should be performed to better assess the size of the effect, or to confirm our findings. /EXPTL Therapy:/ Rats were injected with 10 mg/kg azoxymethane sc weekly for 12 weeks to induce colorectal cancers. At 20 weeks, subtotal colectomies were performed on rats with colorectal tumors and without peritoneal implants or liver metastases. At the time of surgery, a cut portion of the tumor was placed in the abdomen for 30 minutes; the rats then randomly received peritoneal irrigation with chlorhexidine, or sterile water (control). Eight weeks postoperatively a necropsy was performed. At that time, obvious and suspected recurrences and the anastomotic area were sampled for histologic evaluation. Significant differences were seen with chlorhexidine vs. water for gross tumor (P=0.05) and microscopic tumor (P<0.05). Drug Warnings For external use only: For external use only. Keep out of eyes, ears, and mouth. Chlorhexidine gluconate should not be used as a preoperative skin preparation of the face or head. Misuse of products containing chlorhexidine gluconate has been reported to cause serious and permanent eye injury when it has been permitted to enter and remain in the eye during surgical procedures. If chlohexidine gluconate should contact these areas, rinse out promptly and thoroughly with cold water. Avoid contact with neninges. Do not use in genital area. /Chlorhexidine gluconate-topical/ Sensitivity: Chlorhexidine gluconate should not be used by persons who have a sensitivity to it or its components. Hypersensitivity reactions: Irritation, sensitization, and generalized allergic reactions have been reported with chlorhexidine-containing products, especially in the genital areas. If adverse reactions occur and last more than 72 hr, discontinue use immediately and, if severe, contact a health care provider. Deafness: Chlorhexidine gluconate has been reported to cause deafness when instilled in the middle ear through perforate ear drums. /Chlorhexidine gluconate-topical/ For more Drug Warnings (Complete) data for CHLORHEXIDINE (8 total), please visit the HSDB record page. Pharmacodynamics Chlorhexidine is a broad-spectrum antimicrobial with demonstrated activity against both gram-positive and gram-negative bacteria, yeasts, and viruses. Antimicrobial activity is dose-dependent - chlorhexidine is bacteriostatic at lower concentrations (0.02%-0.06%) and bactericidal at higher concentrations (>0.12%). Pharmacokinetic studies of oral chlorhexidine rinses indicate that approximately 30% of the active ingredient is retained in the mouth following rinsing, which is subsequently slowly released into oral fluids. This ability to adsorb to dentine, shared with tetracycline antibiotics such as [doxycycline], is known as "substantivity" and is the result of chlorhexidine's positive charge - it is likely that this substantivity plays at least some role in chlorhexidine's antimicrobial activity, as its persistence on surfaces such as dentine prevent microbial colonization. Dental chlorhexidine rinses may result in staining of oral surfaces, such as teeth. This effect is not ubiquitous and appears to be more significant with extended therapy (i.e. up to 6 months) - nevertheless, patients for whom oral staining is unacceptable should use chlorhexidine rinse with caution and for the shortest effective interval. Allergic reactions to chlorhexidine have been associated with the development of anaphylaxis. |
| 分子式 |
C22H32CL4N10
|
|---|---|
| 分子量 |
578.3685
|
| 精确质量 |
576.156
|
| CAS号 |
3697-42-5
|
| 相关CAS号 |
Chlorhexidine (digluconate);18472-51-0;Chlorhexidine;55-56-1;Chlorhexidine diacetate;56-95-1;Chlorhexidine-d8 dihydrochloride;2012598-75-1
|
| PubChem CID |
9571016
|
| 外观&性状 |
Crystals from methanol
Solid |
| 沸点 |
699.3ºC at 760mmHg
|
| 熔点 |
255-262 ºC
|
| 闪点 |
376.7ºC
|
| LogP |
7.887
|
| tPSA |
167.58
|
| 氢键供体(HBD)数目 |
8
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
13
|
| 重原子数目 |
36
|
| 分子复杂度/Complexity |
649
|
| 定义原子立体中心数目 |
0
|
| SMILES |
ClC1C([H])=C([H])C(=C([H])C=1[H])N([H])/C(/N([H])[H])=N/C(/N([H])[H])=N/C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])/N=C(\N([H])[H])/N=C(\N([H])[H])/N([H])C1C([H])=C([H])C(=C([H])C=1[H])Cl.Cl[H].Cl[H]
|
| InChi Key |
WJLVQTJZDCGNJN-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C22H30Cl2N10.2ClH/c23-15-5-9-17(10-6-15)31-21(27)33-19(25)29-13-3-1-2-4-14-30-20(26)34-22(28)32-18-11-7-16(24)8-12-18;;/h5-12H,1-4,13-14H2,(H5,25,27,29,31,33)(H5,26,28,30,32,34);2*1H
|
| 化学名 |
(1E)-2-[6-[[amino-[(E)-[amino-(4-chloroanilino)methylidene]amino]methylidene]amino]hexyl]-1-[amino-(4-chloroanilino)methylidene]guanidine;dihydrochloride
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~20.83 mg/mL (~36.02 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (3.60 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (3.60 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (3.60 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7290 mL | 8.6450 mL | 17.2900 mL | |
| 5 mM | 0.3458 mL | 1.7290 mL | 3.4580 mL | |
| 10 mM | 0.1729 mL | 0.8645 mL | 1.7290 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。