| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
DNA Alkylator
|
|---|---|
| 体外研究 (In Vitro) |
通过增强 DNA 分子的互补链,苯丁酸氮芥通过引起碱化诱导的交叉反应来抑制肿瘤细胞的生长。 Chlorambucil (0、2.5、5、10 μM) 显着增强 Raji 细胞中 DR4 和 DR5 mRNA 的表达,同时对它们有轻微的阻断作用。用 10 μM 苯丁酸氮芥和 80 ng/mL 肿瘤因子相关磷酸配体 (TRAIL) 处理的 Rati 细胞表达 DR4 和 DR5 mRNA。 Chlorambucil 是高浓度的 DNA 烷化剂,低浓度时是核蛋白合成(特别是蛋白质组生产)的配体。虽然长期维持治疗与导致后续癌症的 p53 基因改变有关,但增加剂量与更高的细胞绝育频率有关 [4]。
|
| 体内研究 (In Vivo) |
艾氏腹水癌可用左旋咪唑(5mg/kg)与苯丁酸氮芥(0.2mg/kg,po)联合治疗,可增加抗癌效果,提高疾病的透明性和抗癌率。小鼠的肾脏和肝脏受到其负面影响[2]。
|
| 细胞实验 |
处于对数生长期的培养细胞被胰蛋白酶分解成单细胞悬液后,以每孔 1000 个细胞的密度接种到 96 孔板中。将板设置在 37°C、5% CO2 室中。贴壁生长 24 小时后,用 0、20、40 和 80 ng/mL 的 TRAIL 或 0、2.5、5 和 10 μM 苯丁酸氮芥处理 48 小时。每孔加入 10 μL CCK-8 试剂后,将混合物在 37°C 下孵育 4 小时。随后,酶标仪测量 450 nm 处的吸光度值。对于每个治疗组,运行六个平行样本。细胞增殖率(%)=对照组平均值×100%/实验组平均值[1]。
|
| 动物实验 |
Mice: Swiss female mice are split into five groups at random (20 mice in each group). Group 1 is maintained as the control group. Group 2 is administered 2.5 × 106 Ehrlich ascites carcinoma cells intraperitoneally. Group 3 is given oral treatment with chlorambucil at a dose of 0.2 mg/kg body weight. Group 4 is given oral treatment with levamisole at a dose of 5 mg/kg body weight. Group 5 is given daily oral treatment with a combination of chlorambucil and levamisole, administered through a bent stainless steel stomach tube[2].
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Chlorambucil is extensively metabolized in the liver primarily to phenylacetic acid mustard. The pharmacokinetic data suggests that oral chlorambucil undergoes rapid gastrointestinal absorption and plasma clearance and that it is almost completely metabolized, having extremely low urinary excretion. Chlorambucil is rapidly and completely absorbed from the GI tract. Following single oral doses of 0.6-1.2 mg/kg, peak plasma concentrations of chlorambucil are reached within 1 hour. In a limited number of patients given a single oral dose of chlorambucil 0.2 mg/kg, an average peak plasma chlorambucil concentration of 492 ng/mL (adjusted to a dose of 12 mg) was reached at about 0.83 hours, and a mean peak plasma concentration of phenylacetic acid mustard (the major metabolite of chlorambucil) of 306 ng/mL (adjusted to a chlorambucil dose of 12 mg) occurred at approximately 1.9 hours. The area under the plasma concentration-time curve (AUC) of phenylacetic acid mustard was about 1.36 times greater than the AUC of chlorambucil. In a study of 12 patients given single oral doses of 0.2 mg/kg of chlorambucil, the mean dose (12 mg) adjusted (+/-SD) plasma chlorambucil Cmax was 492 +/- 160 ng/mL, the AUC was 883 +/- 329 ng.hr/mL, t1/2 was 1.3 +/- 0.5 hours, and the tmax was 0.83 +/- 0.53 hours. For the major metabolite, phenylacetic acid mustard, the mean dose (12 mg) adjusted (+/- SD) plasma Cmax was 306 +/- 73 ng/mL, the AUC was 1204 +/- 285 ng.h/mL, the t1/2 was 1.8 +/- 0.4 hours, and the tmax was 1.9 +/- 0.7 hours. Chlorambucil and its metabolites are extensively bound to plasma and tissue proteins. In vitro, chlorambucil is 99% bound to plasma proteins, specifically albumin. For more Absorption, Distribution and Excretion (Complete) data for CHLORAMBUCIL (12 total), please visit the HSDB record page. Metabolism / Metabolites Chlorambucil undergoes rapid metabolism to phenylacetic acid mustard, the major metabolite, and the combined chlorambucil and phenylacetic acid mustard urinary excretion is extremely low - less than 1% in 24 hours. Chlorambucil and its major metabolite spontaneously degrade in vivo forming monohydroxy and dihydroxy derivatives. Chlorambucil is extensively metabolized in rodents by monochloroethylation and by beta oxidation, forming the phenylacetic acid derivative, which also has anticancer activity. Ten metabolites of chlorambucil were isolated, most of which were phenylacetic acid & benzoic acid derivatives. Route of Elimination: Chlorambucil is extensively metabolized in the liver primarily to phenylacetic acid mustard. The pharmacokinetic data suggests that oral chlorambucil undergoes rapid gastrointestinal absorption and plasma clearance and that it is almost completely metabolized, having extremely low urinary excretion. Half Life: 1.5 hours Biological Half-Life 1.5 hours In a study of 12 patients given single oral doses of 0.2 mg/kg of chlorambucil, ... t1/2 was 1.3 +/- 0.5 hours, and the tmax was 0.83 +/- 0.53 hours. For the major metabolite, phenylacetic acid mustard, ... the t1/2 was 1.8 +/- 0.4 hours, and the tmax was 1.9 +/- 0.7 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Alkylating agents work by three different mechanisms: 1) attachment of alkyl groups to DNA bases, resulting in the DNA being fragmented by repair enzymes in their attempts to replace the alkylated bases, preventing DNA synthesis and RNA transcription from the affected DNA, 2) DNA damage via the formation of cross-links (bonds between atoms in the DNA) which prevents DNA from being separated for synthesis or transcription, and 3) the induction of mispairing of the nucleotides leading to mutations. Hepatotoxicity Chlorambucil therapy is associated with a low rate of serum enzyme elevations, but these are generally mild and self limited, not requiring dose adjustment. Rare instances of clinically apparent acute liver injury attributed to chlorambucil have been reported. The onset of symptoms was within 2 to 6 weeks of starting chlorambucil and the typical enzyme pattern was cholestatic. Some cases have had features of hypersensitivity (rash, fever), and liver injury has recurred upon rechallenge. This form of liver injury is rare and resembles the idiosyncratic acute liver injury due to cyclophosphamide. Chlorambucil has not been linked specifically to sinusoidal obstruction syndrome, but it is not used in high doses in neoplastic disease or in conditioning regimens for hematopoietic cell transplantation, situations in which alkylating agents are commonly associated with this complication. Chlorambucil therapy has also been linked to hypersensitivity reactions and severe cutaneous adverse events such as Stevens Johnson syndrome and toxic epidermal necrolysis, both of which can be accompanied by serum enzyme elevations and hepatitis. Likelihood score: D (possible rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of chlorambucil during breastfeeding. Most sources consider breastfeeding to be contraindicated during maternal cytotoxic antineoplastic drug therapy, especially alkylating agents such as chlorambucil. Chemotherapy may adversely affect the normal microbiome and chemical makeup of breastmilk. Women who receive chemotherapy during pregnancy are more likely to have difficulty nursing their infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Telephone follow-up study was conducted on 74 women who received cancer chemotherapy at one center during the second or third trimester of pregnancy to determine if they were successful at breastfeeding postpartum. Only 34% of the women were able to exclusively breastfeed their infants, and 66% of the women reported experiencing breastfeeding difficulties. This was in comparison to a 91% breastfeeding success rate in 22 other mothers diagnosed during pregnancy, but not treated with chemotherapy. Other statistically significant correlations included: 1. mothers with breastfeeding difficulties had an average of 5.5 cycles of chemotherapy compared with 3.8 cycles among mothers who had no difficulties; and 2. mothers with breastfeeding difficulties received their first cycle of chemotherapy on average 3.4 weeks earlier in pregnancy. None of the patients in the study received chlorambucil. Protein Binding 99% Interactions Because normal defense mechanisms may be suppressed by chlorambucil therapy, concurrent use with a live virus vaccine may potentiate the replication of the vaccine virus, may increase the side/adverse effects of the vaccine virus, and/or may decrease the patient's antibody response to the vaccine; immunization of these patients should be undertaken only with extreme caution after careful review of the patient's hematologic status and only with the knowledge and consent of the physician managing the chlorambucil therapy. The interval between discontinuation of medication that cause immunosuppression and restoration of the patient's ability to respond to the vaccine depends on the intensity and type of immunosuppression-causing medications used, the underlying disease, and other factors; estimates vary from 3 months to 1 year. Patients with leukemia in remission should not receive live virus vaccine until at least 3 months after their last chemotherapy. In addition, immunization with oral polio-virus vaccine should be postponed in persons in close contact with the patient, especially family members. These medications /tricyclic antidepressants and possibly, structurally related compounds such as cyclobenzaprine, haloperidol, loxapine, maprotiline, molindone, monoamine oxidase inhibitors including furazolidone, procarbazine, and selegiline, phenothiazines, pimozide, thioxanthenes/ may lower the seizure threshold and increase the risk of chlorambucil-induced seizures. Leukopenic and/or thrombocytopenic effects of chlorambucil may be increased with concurrent or recent therapy /with blood dyscrasia-causing medications/ if these medications cause the same effects; dosage adjustment of chlorambucil, if necessary, should be based on blood counts. Additive bone marrow depression may occur; dosage reduction may be required when two or more bone marrow depressants, including radiation, are used concurrently or consecutively /with chlorambucil/. For more Interactions (Complete) data for CHLORAMBUCIL (8 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mouse sc 115 mg/kg LD50 Mouse ip 30 mg/kg LD50 Mouse oral 101 mg/kg LD50 Rat ip 14 mg/kg LD50 Rat oral 76 mg/kg |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Antineoplastic Agents, Alkylating; Carcinogens Chlorambucil is indicated in the treatment of chronic lymphatic (lymphocytic) leukemia, malignant lymphomas including lymphosarcoma, giant follicular lymphoma, and Hodgkin's disease. It is not curative in any of these disorders but may produce clinically useful palliation. /Include in US product label/ Chlorambucil is considered by many clinicians to be the drug of choice for the treatment of (Waldenstrom's) macroglobulinemia. /Not included in US product label/ Chlorambucil has also been used effectively with prednisone in the treatment of children with minimal-change nephrotic syndrome (lipoid nephrosis, idiopathic nephrotic syndrome of childhood) who have frequent relapses, require corticosteroid therapy to maintain remissions, or whose disease is resistant to steroid therapy. In most of these children, chlorambucil and prednisone therapy has induced long-term remissions and decreased the frequency of relapses. Although this type of nephrotic syndrome only occasionally occurs in adults, it is treated similarly. /Not included in US product label/ For more Therapeutic Uses (Complete) data for CHLORAMBUCIL (9 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ Chlorambucil can severely suppress bone marrow function. Chlorambucil is a carcinogen in humans. Chlorambucil is probably mutagenic and teratogenic in humans. Chlorambucil produces human infertility. Chlorambucil is contraindicated in patients with known hypersensitivity to the drug or in patients whose disease was resistant to prior therapy with the drug. The manufacturer states that there may be cross-sensitivity between chlorambucil and other alkylating agents manifested as rash. Chlorambucil should be discontinued promptly in patients who develop skin reactions. Adverse hematologic effects are the major and dose-limiting effects of chlorambucil. In usual doses, myelosuppression generally occurs gradually, is moderate in severity, and is usually reversible following discontinuance of the drug. Leukopenia, resulting from neutropenia and slowly progressive lymphopenia, occurs in many patients receiving chlorambucil. Thrombocytopenia and anemia may also occur. Chlorambucil appears to be relatively free of adverse GI effects unless single doses of 20 mg or more are administered. Adverse GI effects include nausea, vomiting, gastric discomfort or abdominal pain, anorexia, and diarrhea. Adverse GI effects are usually mild, last less than 24 hours, and disappear despite continued treatment; however, nausea and weakness have persisted up to 7 days in some patients following a single, high dose of the drug. If necessary, nausea and vomiting may usually be controlled with antiemetics. Oral ulceration has also been reported. For more Drug Warnings (Complete) data for CHLORAMBUCIL (25 total), please visit the HSDB record page. Pharmacodynamics Chlorambucil is an antineoplastic in the class of alkylating agents that is used to treat various forms of cancer. Alkylating agents are so named because of their ability to add alkyl groups to many electronegative groups under conditions present in cells. They stop tumor growth by cross-linking guanine bases in DNA double-helix strands - directly attacking DNA. This makes the strands unable to uncoil and separate. As this is necessary in DNA replication, the cells can no longer divide. In addition, these drugs add methyl or other alkyl groups onto molecules where they do not belong which in turn inhibits their correct utilization by base pairing and causes a miscoding of DNA. Alkylating agents are cell cycle-nonspecific. Alkylating agents work by three different mechanisms all of which achieve the same end result - disruption of DNA function and cell death. |
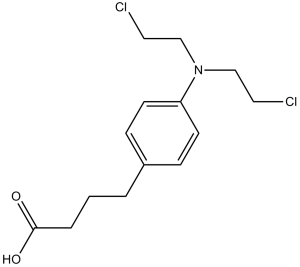
| 分子式 |
C14H19CL2NO2
|
|
|---|---|---|
| 分子量 |
304.21
|
|
| 精确质量 |
303.079
|
|
| 元素分析 |
C, 55.27; H, 6.30; Cl, 23.31; N, 4.60; O, 10.52
|
|
| CAS号 |
305-03-3
|
|
| 相关CAS号 |
|
|
| PubChem CID |
2708
|
|
| 外观&性状 |
Pale brown to brown solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
460.1±40.0 °C at 760 mmHg
|
|
| 熔点 |
64ºC
|
|
| 闪点 |
232.1±27.3 °C
|
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
|
| 折射率 |
1.570
|
|
| LogP |
3.1
|
|
| tPSA |
40.54
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
9
|
|
| 重原子数目 |
19
|
|
| 分子复杂度/Complexity |
250
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O=C(O)CCCC1=CC=C(N(CCCl)CCCl)C=C1
|
|
| InChi Key |
JCKYGMPEJWAADB-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C14H19Cl2NO2/c15-8-10-17(11-9-16)13-6-4-12(5-7-13)2-1-3-14(18)19/h4-7H,1-3,8-11H2,(H,18,19)
|
|
| 化学名 |
4-[4-[bis(2-chloroethyl)amino]phenyl]butanoic acid
|
|
| 别名 |
CB-1348; WR-139013; CB1348; WR139013; CB 1348; WR 139013; chlorambucilum; chloraminophen; Chlorbutin; chlorbutine; chlorbutinum; chloroambucil; chlorobutin; chlorobutine; Leukersan; Leukoran; Lympholysin; phenylbutyric acid nitrogen mustard; US brand names: Ambochlorin; Amboclorin; Leukeran; Linfolizin. Foreign brand names: Altichlorambucil; Chloraminophene; Linfolysin.
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.22 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (8.22 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (8.22 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.2872 mL | 16.4360 mL | 32.8720 mL | |
| 5 mM | 0.6574 mL | 3.2872 mL | 6.5744 mL | |
| 10 mM | 0.3287 mL | 1.6436 mL | 3.2872 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02242942 | Active Recruiting |
Drug: Chlorambucil Drug: Venetoclax |
Lymphocytic Leukemia, Chronic | Hoffmann-La Roche | December 31, 2014 | Phase 3 |
| NCT03462719 | Active Recruiting |
Drug: Ibrutinib Drug: Venetoclax |
Leukemia, Lymphocytic, Chronic, B-Cell |
Janssen Research & Development, LLC |
April 17, 2018 | Phase 3 |
| NCT04075292 | Active Recruiting |
Drug: Acalabrutinib Drug: Rituximab |
Untreated Chronic Lymphocytic Leukemia |
AstraZeneca | January 20, 2020 | Phase 3 |
| NCT02475681 | Active Recruiting |
Drug: Acalabrutinib Drug: Obinutuzumab |
Chronic Lymphocytic Leukemia | Acerta Pharma BV | June 26, 2015 | Phase 3 |
| NCT04692740 | Active Recruiting |
Drug: Chlorambucil, Oral, 2 Mg | Pancreatic Ductal Adenocarcinoma | Michele Reni | December 18, 2020 | Phase 2 |