| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| 50g |
|
||
| Other Sizes |
|
| 靶点 |
β-lactam
|
|---|---|
| 体外研究 (In Vitro) |
用头孢哌酮(气雾剂处理,肺匀浆中终浓度为 60 μg/mL)治疗的粒细胞减少小鼠可预防急性铜绿假单胞菌肺炎[2]。
|
| 体内研究 (In Vivo) |
在本研究中,我们试图确定主要参与β-内酰胺类抗生素分子量依赖性胆汁排泄的膜渗透过程。文献检索表明,分子量阈值主要在穿过胆管膜的运输过程中起作用。我们证实,在遗传上缺乏多药耐药相关蛋白(Mrp)2的卫材高胆红素血症大鼠中,模型胆汁排泄型头孢菌素头孢哌酮的胆汁清除率降低到对照组的10%,表明Mrp2作为小管膜上的外排转运蛋白起着主要作用。在转染了大鼠Mrp2的Sf9细胞的膜囊泡中证实了几种头孢菌素的ATP依赖性摄取,包括头孢哌酮、头孢哌拉酮、头孢匹胺和头孢曲松,所有这些主要排泄到胆汁中。头孢菌素对Mrp2介导的转运的抑制效力和表达Mrp2的囊泡对头孢菌素的摄取都是分子量依赖性的,这表明Mrp2是参与分子量依赖的胆汁排泄的主要转运蛋白之一。一项对转染癌症耐药蛋白(Bcrp)的Sf9细胞膜囊泡的摄取研究表明,Bcrp接受头孢哌酮、头孢布培酮、头孢匹胺、头孢替坦、头孢曲松、头孢噻安、头孢曼吲哚和头孢唑林作为底物,并且Bcrp介导的转运也是分子量依赖性的,这表明Bcrp也有助于大鼠β-巴坦抗生素的分子量依赖的胆汁排泄[3]。
|
| 酶活实验 |
头孢哌酮是一种新型的半合成头孢菌素,具有广谱抗菌活性。它对革兰氏阳性细菌的活性与头孢唑林和头孢孟多相当,对大肠杆菌、肺炎克雷伯菌、变形杆菌、铜绿假单胞菌、弗氏柠檬酸杆菌、阴沟肠杆菌和粘质沙雷氏菌等革兰氏阴性杆菌的活性比头孢唑林、头孢孟多更高。头孢哌酮对铜绿假单胞菌的活性比头孢唑林和头孢孟多高出200倍以上,这一点尤其显著。与其他β-内酰胺类抗生素一样,头孢哌酮的最低抑菌浓度和最低杀菌浓度之间只有很小的差异,随着接种量的增加,活性显著降低。向试验培养基中加入人血清不会显著改变活性。头孢哌酮对革兰氏阴性菌产生的β-内酰胺酶的水解相对稳定。头孢哌酮被头孢菌素酶水解的相对速率为7.0至0.01,参考头孢丙啶水解(碱,100)。头孢哌酮对各种类型的青霉素酶也比青霉素G和头孢噻啶更稳定[1]。
|
| 动物实验 |
The pharmacokinetics of cefoperazone in normal subjects, and in patients with hepatic and renal dysfunction are reviewed. After intravenous administration of 2 g of cefoperazone, levels in serum ranged from 202 to 375 microgram/ml depending on the period of drug administration. After intramuscular injection of 2 g of cefoperazone, the mean peak serum level was 111 microgram/ml at 1.5 hours. At 12 hours after dosing, mean serum levels were still 2 to 4 microgram/ml. Cefoperazone was 90% bound to serum proteins. The apparent volume of distribution was 10 to 13L. The half-life of the drug varied from 1.6 to 2.4 hours; serum clearance was between 75 and 96 ml/min. Urinary excretion was rapid, but only 15 to 36% of the cefoperazone dose was recovered in the urine. Renal clearance ranged from 14 to 25 ml/min. Urine levels of cefoperazone in excess of 32 microgram/ml were maintained for at least 12 hours. Biliary levels of cefoperazone were many-fold higher than serum levels; peak bile concentrations from 675 to 6000 microgram/ml were obtained. Severe hepatic dysfunction was associated with a 2- to 4-fold increase in the half-life of cefoperazone. In patients with relatively complete biliary obstruction, over 90% of the dose was recovered in the urine. In contrast, the serum kinetics of cefoperazone were not significantly altered in patients with renal impairment. The human pharmacology of cefoperazone is similar to cephazolin in terms of serum concentrations, half-life, protein binding, and apparent volume of distribution, but markedly different in terms of biliary and renal excretion. Since biliary excretion is normally the primary route of cefoperazone elimination, dosage modification should only be required in the presence of severe biliary obstruction or concomitant renal and hepatic dysfunction.
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Cefoperazone is excreted mainly in the bile. Metabolism / Metabolites No significant quanitity of metabolites have been identified in urine. Biological Half-Life The mean serum half-life is approximately 2.0 hours, independent of the route of administration. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Cefoperazone is no longer marketed in the United States. Limited information indicates cefoperazone produces low levels in milk that are not expected to cause adverse effects in breastfed infants. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush have been reported with cephalosporins, but these effects have not been adequately evaluated. Cefoperazone is acceptable in nursing mothers. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding The degree of reversible protein binding varies with the serum concentration from 93% at 25 mcg/mL to 90% at 250 mcg/mL and 82% at 500 mcg/mL. Cefotetan is 88% plasma protein bound. |
| 参考文献 | |
| 其他信息 |
Pharmacodynamics
Cefoperazone is a third generation cephalosporin antibiotic. Cefoperazone exerts its bactericidal effect by inhibiting the bacterial cell wall synthesis |
| 分子式 |
C25H27N9O8S2
|
|---|---|
| 分子量 |
645.6674
|
| 精确质量 |
645.142
|
| 元素分析 |
C, 46.51; H, 4.22; N, 19.52; O, 19.82; S, 9.93
|
| CAS号 |
62893-19-0
|
| 相关CAS号 |
Cefoperazone sodium salt;62893-20-3;Cefoperazone-d5;2410425-70-4;Cefoperazone dihydrate;113826-44-1
|
| PubChem CID |
44187
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.8±0.1 g/cm3
|
| 熔点 |
169-171ºC
|
| 折射率 |
1.819
|
| LogP |
1.43
|
| tPSA |
270.86
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
13
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
44
|
| 分子复杂度/Complexity |
1250
|
| 定义原子立体中心数目 |
3
|
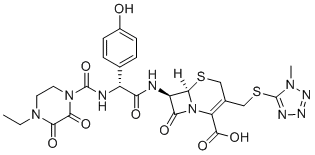
| SMILES |
S1C([H])([H])C(C([H])([H])SC2=NN=NN2C([H])([H])[H])=C(C(=O)O[H])N2C([C@]([H])([C@@]12[H])N([H])C([C@@]([H])(C1C([H])=C([H])C(=C([H])C=1[H])O[H])N([H])C(N1C(C(N(C([H])([H])C([H])([H])[H])C([H])([H])C1([H])[H])=O)=O)=O)=O)=O
|
| InChi Key |
GCFBRXLSHGKWDP-XCGNWRKASA-N
|
| InChi Code |
InChI=1S/C25H27N9O8S2/c1-3-32-8-9-33(21(39)20(32)38)24(42)27-15(12-4-6-14(35)7-5-12)18(36)26-16-19(37)34-17(23(40)41)13(10-43-22(16)34)11-44-25-28-29-30-31(25)2/h4-7,15-16,22,35H,3,8-11H2,1-2H3,(H,26,36)(H,27,42)(H,40,41)/t15-,16-,22-/m1/s1
|
| 化学名 |
(6R,7R)-7-((R)-2-(4-ethyl-2,3-dioxopiperazine-1-carboxamido)-2-(4-hydroxyphenyl)acetamido)-3-(((1-methyl-1H-tetrazol-5-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
|
| 别名 |
T1551; T-1551; T 1551; Trade name: Cefobid; Cefozon; C06883; C-06883; C 06883 D07645; D-07645; D 07645; Cefobid; Cefoperazono; Cefoperazonum; Peracef; Cefoperazone acid; Cefoperazone (Cefobid);
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 100 mg/mL (~154.88 mM )
H2O : ~0.1 mg/mL (~0.15 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (3.87 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (3.87 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (3.87 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 30% propylene glycol+ 5% Tween 80+ 65% D5W: 30mg/ml (46.46mM) 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5488 mL | 7.7439 mL | 15.4878 mL | |
| 5 mM | 0.3098 mL | 1.5488 mL | 3.0976 mL | |
| 10 mM | 0.1549 mL | 0.7744 mL | 1.5488 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05742295 | COMPLETED | Drug: Vitamin K | Antibiotic Side Effect | Helwan University | 2023-02-25 | Phase 4 |
| NCT05654090 | ACTIVE, NOT RECRUITING | Drug: cefoperazone sodium and sulbactam sodium | Infectious Diseases | Yung Shin Pharm. Ind. Co., Ltd. | 2022-08-25 | Phase 4 |
| NCT05535309 | COMPLETED | Drug: cefoperazone sulbactam sodium | Risk Factors | Qianfoshan Hospital | 2021-11-02 | |
| NCT00463762 | WITHDRAWN | Drug: CP-75385-02 Cefoperazone/sulbactam |
Abscess, Intra-Abdominal Appendicitis Cholecystitis Peritonitis Wound Infections |
Pfizer | 2007-05 | |
| NCT01992198 | UNKNOWN STATUS | Drug: cefoperazone + metronidazole Procedure: oral care by chlorhexidine gluconate Procedure: enteral nutrition |
Pancreatitis,Acute Necrotizing | Erzhen Chen | 2012-07 | Phase 4 |